We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Cassandra Marsh/Sandbox 2
From Proteopedia
(Difference between revisions)
| Line 33: | Line 33: | ||
===Potassium Binding Site=== | ===Potassium Binding Site=== | ||
| - | There are two potassium ions in HDAC8. While their exact function is unknown, these ions do increase the catalytic activity of the enzyme overall. The potassium ion closest to the active site becomes a <scene name='81/812841/Potassium_binding_site/4'>Potassium Binding Site</scene> | + | There are two potassium ions in HDAC8. While their exact function is unknown, these ions do increase the catalytic activity and stability of the enzyme overall. The potassium ion closest to the active site becomes a <scene name='81/812841/Potassium_binding_site/4'>Potassium Binding Site</scene>. The potassium ion octahedrally coordinates with the side chain oxygen of S199 and D176 and the backbone oxygen of D176, D178, H180 and L200 <ref name="Chen">PMID:25060069</ref>. Because the second potassium ion is about 20 Å from the catalytic center, this only regulates the enzymatic activity by an allosteric effect <ref name="Chen" />. |
| + | |||
| + | |||
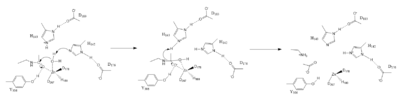
==Deacetylation== | ==Deacetylation== | ||
Revision as of 01:26, 7 April 2019
Histone Deacetylase 8 (HDAC 8), H. sapiens
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Histones | Learn Science at Scitable https://www.nature.com/scitable/definition/histone-histones-57
- ↑ 2.0 2.1 2.2 2.3 2.4 Vannini A, Volpari C, Gallinari P, Jones P, Mattu M, Carfi A, De Francesco R, Steinkuhler C, Di Marco S. Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8-substrate complex. EMBO Rep. 2007 Sep;8(9):879-84. Epub 2007 Aug 10. PMID:17721440
- ↑ 3.0 3.1 Chen K, Zhang X, Wu YD, Wiest O. Inhibition and mechanism of HDAC8 revisited. J Am Chem Soc. 2014 Aug 20;136(33):11636-43. doi: 10.1021/ja501548p. Epub 2014, Aug 7. PMID:25060069 doi:http://dx.doi.org/10.1021/ja501548p
Student Contributors
- Cassandra Marsh
- Courtney Brown
- Carolyn Hurdle