User:Asif Hossain/Sandbox 1
From Proteopedia
| Line 17: | Line 17: | ||
===Key Residues=== | ===Key Residues=== | ||
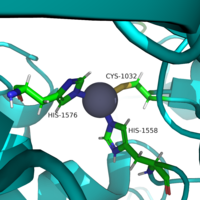
The <scene name='81/811085/Active_site/6'>active site</scene> of HDAC8 is composed of 2 catalytic dyads: Asp183/H143 and Asp176/His142, which activate the catalytic water nucleophile. A Tyr306, through mutation to Phe in the pdb file 2v5w (modeled in the overall view) was observed to render the protein mostly inactive. Thus, it has been hypothesized that the this residue is critical to transition state stabilization with the Zn2+ ion. | The <scene name='81/811085/Active_site/6'>active site</scene> of HDAC8 is composed of 2 catalytic dyads: Asp183/H143 and Asp176/His142, which activate the catalytic water nucleophile. A Tyr306, through mutation to Phe in the pdb file 2v5w (modeled in the overall view) was observed to render the protein mostly inactive. Thus, it has been hypothesized that the this residue is critical to transition state stabilization with the Zn2+ ion. | ||
| + | |||
| + | [[Image:Triad.png |200px|left|thumb|Similar to many serine and zinc proteases, HDAC8 uses a mechanism with a "catalytic triad." Instead of the Asp-His-Ser, HDAC8 uses the His to coordinate a H2O nucleophile.]] | ||
===Binding Pocket=== | ===Binding Pocket=== | ||
| Line 24: | Line 26: | ||
===Asp 101=== | ===Asp 101=== | ||
At the rim of the active site, <scene name='81/811084/Asp101/5'>Asp101</scene> is involved in two hydrogen bonds between its own carbonyl Os and two consecutive amide Hs of incoming <scene name='81/811084/Ligand/7'>peptide-derived ligand</scene>. This forces the ligand to assume a cis-conformation. In addition, extensive interactions among many other polar atoms near the rim of the active site help keep the ligand lodged in the hydrophobic tunnel.** | At the rim of the active site, <scene name='81/811084/Asp101/5'>Asp101</scene> is involved in two hydrogen bonds between its own carbonyl Os and two consecutive amide Hs of incoming <scene name='81/811084/Ligand/7'>peptide-derived ligand</scene>. This forces the ligand to assume a cis-conformation. In addition, extensive interactions among many other polar atoms near the rim of the active site help keep the ligand lodged in the hydrophobic tunnel.** | ||
| - | |||
| - | |||
| - | |||
==Mechanism== | ==Mechanism== | ||
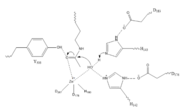
| - | Once bound to the binding pocket through interactions with Asp101, Phe153, and Phe208, the water molecule nucleophilically attacks the carbonyl carbon of the ε-amino-lysine sidechain of N-terminal core of histone proteins. This water molecule is recruited and stabilized by two catalytic dyads. The first dyad consists of His143 and Asp183. Asp183 interacts with His143 to shift electron density so that His143 may act as a general base to remove a proton from water. The second catalytic dyad consists of His142 and Asp176 and stabilizes the now deprotonated water molecule. A Zn2+ ion also makes the water more nucleophilic by drawing positive character away from the water. The tetrahedral intermediate is stabilized by the Zn2+ ion as well as Tyr306. The amine group of the histones lysine residue acts as a general base and deprotonates His143. This drives the tetrahedral intermediate to collapses and expel the acetyl group to produce an acetate ion and a deacetylated lysine residue.[[Image:Mech1.png]] | + | Once bound to the binding pocket through interactions with Asp101, Phe153, and Phe208, the water molecule nucleophilically attacks the carbonyl carbon of the ε-amino-lysine sidechain of N-terminal core of histone proteins. This water molecule is recruited and stabilized by two catalytic dyads. The first dyad consists of His143 and Asp183. Asp183 interacts with His143 to shift electron density so that His143 may act as a general base to remove a proton from water. The second catalytic dyad consists of His142 and Asp176 and stabilizes the now deprotonated water molecule. A Zn2+ ion also makes the water more nucleophilic by drawing positive character away from the water. The tetrahedral intermediate is stabilized by the Zn2+ ion as well as Tyr306. The amine group of the histones lysine residue acts as a general base and deprotonates His143. This drives the tetrahedral intermediate to collapses and expel the acetyl group to produce an acetate ion and a deacetylated lysine residue. |
| + | [[Image:Mech1.png |thumb|]] | ||
| - | [[Image:Triad.png |200px|left|thumb|Similar to many serine and zinc proteases, HDAC8 uses a mechanism with a "catalytic triad." Instead of the Asp-His-Ser, HDAC8 uses the His to coordinate a H2O nucleophile.]] | ||
Revision as of 21:47, 7 April 2019
Contents |
Histone Deacetylase 8 (HDAC 8)
Introduction
Histone deacetylase 8 (HDAC8) is an enzyme that plays a role in controlling gene expression in Homo sapiens. Specifically, HDAC8 catalyzes the removal of an acetyl group off of the ε-amino-lysine sidechain of N-terminal core of histone proteins. Histones consist of eight monomers to form an octomer complex. In addition, histones are highly basic and have a large positive charge. Since DNA is negatively charged, histones tightly interact with DNA. This prevents transcription factors from accessing DNA, thus decreasing gene expression. Histones regulate gene expression without altering the sequence of DNA making histones epigenetic regulators. This interaction between DNA and histones can be disrupted by Histone acetyltransferase (HAT). HATs catalyzes the addition of an acetyl group onto a histone. The hydrophobicity of the acetyl group prevents the interaction between DNA and histones. This allows transcription factors to access the DNA to increase gene expression. HDAC8 reverses this reaction by catalyzing the removal of these acetyl groups By removing the acetate, the reclaimed positive charge on the lysine sidechain is able to interact with the negative charge on the DNA. As a result, DNA will bind more tightly to the histone protein, repressing transcription and expression.
| |||||||||||
Relevance
Besides controlling the gene regulation through deacetylation of histones, HDAC 8 also regulates the post-transcriptional acetylation status of many non-histone proteins, including transcription factors, chaperones, hormone receptors and signaling molecules. Thus, it has influences on protein stability, protein-protein interactions and protein-DNA interactions. HDAC 8 can therefore affect the regulation of cell proliferation and cell death. These processes are typically being altered in cancer cells and that makes HDAC enzymes an interesting potential target for cancer drugs. HDAC inhibitors have been shown to be promising cancer drug agents in prior research as the HDAC inhibitors cease tumor growth in cancer cells by either making them differentiate or undergo apoptosis. One way, the HDAC inhibitors ceases tumor growth is by the reactivation of the transcription factor, RUNX3, a known tumor suppressor. HDACi increases the acetylation of the protein and as the stability of RUNX3 is dependent on the acetylation status of the protein, the increased acetylation or HDAC inhibition will enhance the protein stability, causing an increase in the anti-tumorous properties of the protein. A number of HDAC inhibitors have been purified from natural sources or synthesized and HDAC inhibitors can be structurally grouped into at least four classes: hydrox-amates, cyclic peptides, aliphatic acids and ben-zamides. The Vorinostat(within the hydrox-amates class) has been FDA-approved for treatment of cancer. The hydrox-amate HDAC inhibitors inhibits the HDAC 8 by competition inhibition by making a favorable bidental interaction with the Zn2+ Ion and makes strong interactions with the Asp101.
References
1. Whitehead, L., Dobler, M. R., Radetich, B., Zhu, Y., Atadja, P. W., Claiborne, T., ... & Shao, W. (2011). Human HDAC isoform selectivity achieved via exploitation of the acetate release channel with structurally unique small molecule inhibitors. Bioorganic & medicinal chemistry, 19(15), 4626-4634.