We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
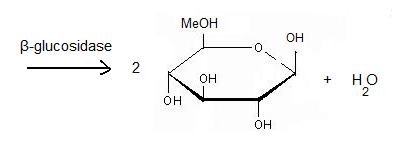
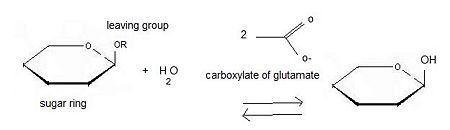
Beta-glucosidase
From Proteopedia
(Difference between revisions)
| Line 80: | Line 80: | ||
==Additional Resources== | ==Additional Resources== | ||
For additional information, see: [[Carbohydrate Metabolism]] | For additional information, see: [[Carbohydrate Metabolism]] | ||
| + | |||
| + | ==3D structures of Beta-glucosidase== | ||
| + | [[Beta-glucosidase 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
| Line 106: | Line 110: | ||
**[[1oif]], [[1od0]] – TmGB catalytic domain - ''Thermotoga maritima''<br /> | **[[1oif]], [[1od0]] – TmGB catalytic domain - ''Thermotoga maritima''<br /> | ||
**[[4gxp]] – TmGB/TrGB<br /> | **[[4gxp]] – TmGB/TrGB<br /> | ||
| - | **[[1ug6]] – | + | **[[1ug6]] – TtGB - ''Thermus thermophilus''<br /> |
| + | **[[4bce]] – TtGB (mutant) <br /> | ||
**[[1hxj]], [[1e1e]] – ZmGB – ''Zea mays''<br /> | **[[1hxj]], [[1e1e]] – ZmGB – ''Zea mays''<br /> | ||
**[[1e4l]] - ZmGB (mutant) <br /> | **[[1e4l]] - ZmGB (mutant) <br /> | ||
| Line 113: | Line 118: | ||
**[[1tr1]] - BpGBB (mutant) - ''Bacillus polymyxa''<br /> | **[[1tr1]] - BpGBB (mutant) - ''Bacillus polymyxa''<br /> | ||
**[[1cbg]] – GB cyanogenic – White clover<br /> | **[[1cbg]] – GB cyanogenic – White clover<br /> | ||
| - | **[[3aiu]] - | + | **[[3aiu]] - ryGB residues 50-568 – rye<br /> |
**[[3ut0]], [[3usz]] – PsGB residues 28-840 – ''Pseudoalteromonas''<br /> | **[[3ut0]], [[3usz]] – PsGB residues 28-840 – ''Pseudoalteromonas''<br /> | ||
**[[3f95]] – PsGB residues 657-840<br /> | **[[3f95]] – PsGB residues 657-840<br /> | ||
| Line 122: | Line 127: | ||
**[[3zyz]], [[3zz1]] – GB – ''Hypocrea jecorina''<br /> | **[[3zyz]], [[3zz1]] – GB – ''Hypocrea jecorina''<br /> | ||
**[[4hz6]] – ubGB – uncultured bacterium<br /> | **[[4hz6]] – ubGB – uncultured bacterium<br /> | ||
| - | **[[4bce]] – GB (mutant) – ''Thermus thermophilus''<br /> | ||
**[[4iib]] – AaGB – ''Aspergillus aculeatus''<br /> | **[[4iib]] – AaGB – ''Aspergillus aculeatus''<br /> | ||
**[[5fjj]] – GB – ''Aspergillus oryzae''<br /> | **[[5fjj]] – GB – ''Aspergillus oryzae''<br /> | ||
| Line 133: | Line 137: | ||
**[[3w53]] – GB – ''Micrococcus antarcticus''<br /> | **[[3w53]] – GB – ''Micrococcus antarcticus''<br /> | ||
**[[4mdo]] – HgGB – ''Humicola grisea''<br /> | **[[4mdo]] – HgGB – ''Humicola grisea''<br /> | ||
| - | **[[3wh5]], [[3u48]], [[3u4a]], [[5gnx]], [[5gny]], [[5gnz]], [[5wka]] – MeGB – ''Metagenome''<br /> | + | **[[3wh5]], [[3u48]], [[3u4a]], [[5gnx]], [[5gny]], [[5gnz]], [[5wka]], [[5ns6]] – MeGB – ''Metagenome''<br /> |
**[[5ayi]], [[5ayb]] – MeGB (mutant)<br /> | **[[5ayi]], [[5ayb]] – MeGB (mutant)<br /> | ||
**[[5ju6]] – GB – ''Talaromyces emersonii''<br /> | **[[5ju6]] – GB – ''Talaromyces emersonii''<br /> | ||
**[[5wug]], [[5wvp]] – GB – ''Paenibacillus barengoltzii''<br /> | **[[5wug]], [[5wvp]] – GB – ''Paenibacillus barengoltzii''<br /> | ||
**[[5xxl]] – BtGB – ''Bacterioides thetaiotaomicron''<br /> | **[[5xxl]] – BtGB – ''Bacterioides thetaiotaomicron''<br /> | ||
| + | **[[5ogz]] – GB – ''Ruminiclostridium thermocellum''<br /> | ||
*Beta-glucosidase complex with sugar | *Beta-glucosidase complex with sugar | ||
| Line 154: | Line 159: | ||
**[[3aht]], [[3ahv]], [[3scq]], [[3scs]], [[4qlk]], [[4qlj]] – JrGB7 (mutant) + saccharide<br /> | **[[3aht]], [[3ahv]], [[3scq]], [[3scs]], [[4qlk]], [[4qlj]] – JrGB7 (mutant) + saccharide<br /> | ||
**[[1oin]] - TmGBA + glucoside<br /> | **[[1oin]] - TmGBA + glucoside<br /> | ||
| + | **[[5oss]] – TmGB + glucoimidazole<br /> | ||
**[[4hz7]], [[4hz8]] - UbGB residues 18-482 (mutant) + glucoside<br /> | **[[4hz7]], [[4hz8]] - UbGB residues 18-482 (mutant) + glucoside<br /> | ||
**[[2zox]], [[2e9l]], [[2e9m]] - hGB cystolic + glucoside<br /> | **[[2zox]], [[2e9l]], [[2e9m]] - hGB cystolic + glucoside<br /> | ||
| Line 199: | Line 205: | ||
**[[1y7v]] - hGB (mutant) + inhibitor<br /> | **[[1y7v]] - hGB (mutant) + inhibitor<br /> | ||
**[[5bx3]] - TxGB + inhibitor<br /> | **[[5bx3]] - TxGB + inhibitor<br /> | ||
| + | **[[5ns8]] – MeGB (mutant) + deoxynojirimycin<br /> | ||
*6-phospho-β-glucosidase | *6-phospho-β-glucosidase | ||
| Line 205: | Line 212: | ||
**[[1up4]] – TmPGB <br /> | **[[1up4]] – TmPGB <br /> | ||
**[[1up6]], [[1up7]] – TmPGB + NAD + G6P<br /> | **[[1up6]], [[1up7]] – TmPGB + NAD + G6P<br /> | ||
| - | **[[3qom]], [[4gze]] – PGB – ''Lactobacillus plantarum''<br /> | + | **[[3qom]], [[4gze]], [[5nav]] – PGB – ''Lactobacillus plantarum''<br /> |
**[[2xhy]] – EcPGB <br /> | **[[2xhy]] – EcPGB <br /> | ||
**[[1h4p]] – PGB I/II – yeast<br /> | **[[1h4p]] – PGB I/II – yeast<br /> | ||
Revision as of 08:18, 8 April 2019
| |||||||||||
3D structures of Beta-glucosidase
Updated on 08-April-2019
References
- ↑ Aguilar M, Gloster TM, Garcia-Moreno MI, Ortiz Mellet C, Davies GJ, Llebaria A, Casas J, Egido-Gabas M, Garcia Fernandez JM. Molecular basis for beta-glucosidase inhibition by ring-modified calystegine analogues. Chembiochem. 2008 Nov 3;9(16):2612-8. PMID:18833549 doi:10.1002/cbic.200800451
- ↑ http://en.wikipedia.org/wiki/B-glucosidase
- ↑ Davies G, Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure. 1995 Sep 15;3(9):853-9. PMID:8535779
- ↑ http://www.ebi.ac.uk/interpro/IEntry?ac=IPR018120#PUB00002205
- ↑ http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/CSA/CSA_Site_Wrapper.pl?pdb=2vrj
- ↑ Davies G, Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure. 1995 Sep 15;3(9):853-9. PMID:8535779
- ↑ http://www.cazy.org/fam/ghf_INV_RET.html#3
- ↑ Dvir H, Harel M, McCarthy AA, Toker L, Silman I, Futerman AH, Sussman JL. X-ray structure of human acid-beta-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 2003 Jul;4(7):704-9. PMID:12792654 doi:10.1038/sj.embor.embor873
- ↑ 9.0 9.1 9.2 Premkumar L, Sawkar AR, Boldin-Adamsky S, Toker L, Silman I, Kelly JW, Futerman AH, Sussman JL. X-ray structure of human acid-beta-glucosidase covalently bound to conduritol-B-epoxide. Implications for Gaucher disease. J Biol Chem. 2005 Jun 24;280(25):23815-9. Epub 2005 Apr 6. PMID:15817452 doi:M502799200
- ↑ Hrmova M, Varghese JN, De Gori R, Smith BJ, Driguez H, Fincher GB. Catalytic mechanisms and reaction intermediates along the hydrolytic pathway of a plant beta-D-glucan glucohydrolase. Structure. 2001 Nov;9(11):1005-16. PMID:11709165
- ↑ Zeev-Ben-Mordehai T, Silman I, Sussman JL. Acetylcholinesterase in motion: visualizing conformational changes in crystal structures by a morphing procedure. Biopolymers. 2003 Mar;68(3):395-406. PMID:12601798 doi:10.1002/bip.10287
- ↑ 12.0 12.1 12.2 12.3 Shaaltiel Y, Bartfeld D, Hashmueli S, Baum G, Brill-Almon E, Galili G, Dym O, Boldin-Adamsky SA, Silman I, Sussman JL, Futerman AH, Aviezer D. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher's disease using a plant cell system. Plant Biotechnol J. 2007 Sep;5(5):579-90. Epub 2007 May 24. PMID:17524049 doi:10.1111/j.1467-7652.2007.00263.x
- ↑ Brumshtein B, Greenblatt HM, Butters TD, Shaaltiel Y, Aviezer D, Silman I, Futerman AH, Sussman JL. Crystal structures of complexes of N-butyl- and N-nonyl-deoxynojirimycin bound to acid beta-glucosidase: insights into the mechanism of chemical chaperone action in Gaucher disease. J Biol Chem. 2007 Sep 28;282(39):29052-8. Epub 2007 Jul 31. PMID:17666401 doi:10.1074/jbc.M705005200
- ↑ Lieberman RL, Wustman BA, Huertas P, Powe AC Jr, Pine CW, Khanna R, Schlossmacher MG, Ringe D, Petsko GA. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007 Feb;3(2):101-7. Epub 2006 Dec 24. PMID:17187079 doi:http://dx.doi.org/10.1038/nchembio850
- ↑ Brumshtein B, Wormald MR, Silman I, Futerman AH, Sussman JL. Structural comparison of differently glycosylated forms of acid-beta-glucosidase, the defective enzyme in Gaucher disease. Acta Crystallogr D Biol Crystallogr. 2006 Dec;62(Pt 12):1458-65. Epub 2006, Nov 23. PMID:17139081 doi:S0907444906038303
- ↑ 16.0 16.1 Brumshtein B, Salinas P, Peterson B, Chan V, Silman I, Sussman JL, Savickas PJ, Robinson GS, Futerman AH. Characterization of gene-activated human acid-beta-glucosidase: crystal structure, glycan composition, and internalization into macrophages. Glycobiology. 2010 Jan;20(1):24-32. Epub 2009 Sep 9. PMID:19741058 doi:10.1093/glycob/cwp138
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Muriel Breteau, Alexander Berchansky, Joel L. Sussman, David Canner