This is a default text for your page Nicholas Bantz/Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
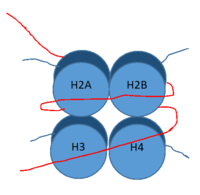
DNA (red) wrapped around histone proteins with histone tails (blue)
LSD-1 is a lysine demethylase. A histone is a blah blah blah and can be seen in Fig. 1.
Structure

LSD1 overall 3D structure: Tower domain (blue), SWIRM domain (yellow), and Oxidase domain (red).
Tower Domain
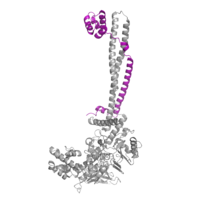
CoRest complex (purple) bound to LSD1 at the Tower domain.
The is a protrusion off the main protein body of LSD-1 comprised of 100 residues, which form 2 𝛂-helices. The longer helix, T𝛂A, is an LSD-1 specific element that has not been found in any other oxidase proteins [3]. The shorter helix, T𝛂B, is very near to the active site of the oxidase domain. In fact, T𝛂B connects directly to helix 𝛂D of the oxidase domain through a highly conserved connector loop. The exact function of the tower domain is not known, but it is proposed to regulate the size of the active site chamber through this . The T𝛂B-𝛂D interaction is responsible for the proper positioning of Phe538, a side chain of 𝛂D that is located in the catalytic chamber. In addition, the T𝛂B-𝛂D interaction positions 𝛂D in the correct manner to provide hydrogen bonding to Tyr761. Tyr761 is positioned in the catalytic chamber very close to the FAD cofactor, and aids in the binding of the lysine substrate [3]. Therefore, the base of the tower domain forms a direct connection to the oxidase domain and plays a crucial role in the shape and catalytic activity of the active site. In fact, removing the tower domain via a mutation resulted in a drastic decrease in catalytic efficiency.(1) The tower domain has also been found to interact with other proteins and complexes, such as CoREST (see Figure 2), as a molecular lever to allosterically regulate the catalytic activity of the active site. (insert other source here) Overall, the exact function of the tower domain has not yet been determined, but it is known to be vital to the catalytic activity of LSD-1.
SWIRM Domain
The domain is a blah blah blah blah.
These are the
Oxidase Domain
The is ALSO a blah blah blah.
The is vitally important because...
Active Site and FAD Cofactor
Mechanism of Active Site

Figure X: Hydride transfer mechanism of LSD-1 active site via FAD cofactor.
This is a picture of the .
These are the different
The is ALSO a blah blah blah.
Inhibition by Tri-Methylated Lysine
Medical Implications
Cancer
Diabetes
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 Stavropoulos P, Blobel G, Hoelz A. Crystal structure and mechanism of human lysine-specific demethylase-1. Nat Struct Mol Biol. 2006 Jul;13(7):626-32. Epub 2006 Jun 25. PMID:16799558 doi:10.1038/nsmb1113



