User:Steve Klimcak/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
<scene name='81/811712/Hydrophobic_pocket/1'>H20phobic Pocket</scene> | <scene name='81/811712/Hydrophobic_pocket/1'>H20phobic Pocket</scene> | ||
== Function == | == Function == | ||
| - | === | + | ===Mechanism=== |
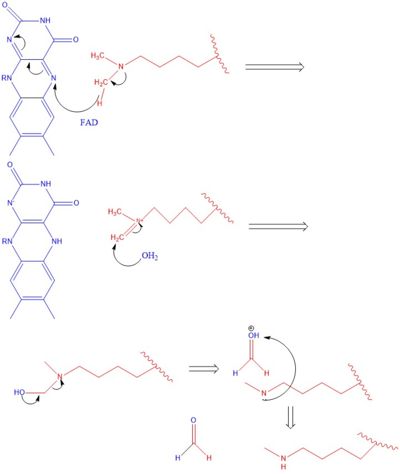
The methylation of lysine via LSD1 occurs through a 2 electron process. In the first step the Flavin Adenine Dinucleotide cofactor initiates a hydride transfer from the one of the two methyl groups bound to the nitrogen at the lysine tail, via a N5 on the flavin group. An imine cation is then formed at the end of the lysine tail to compensate for the loss of hydride. In the next step the imine is hydrolyzed and transitions to an Hemiaminal. This then breaks down easily to formaldehyde and the product lysine. | The methylation of lysine via LSD1 occurs through a 2 electron process. In the first step the Flavin Adenine Dinucleotide cofactor initiates a hydride transfer from the one of the two methyl groups bound to the nitrogen at the lysine tail, via a N5 on the flavin group. An imine cation is then formed at the end of the lysine tail to compensate for the loss of hydride. In the next step the imine is hydrolyzed and transitions to an Hemiaminal. This then breaks down easily to formaldehyde and the product lysine. | ||
[[Image:LSD1 Mech.jpg|400px|right|thumb|Mechanism]] | [[Image:LSD1 Mech.jpg|400px|right|thumb|Mechanism]] | ||
| + | |||
| + | ===Hydrophobic Pocket=== | ||
| + | The hydrophobic pocket located in the active site cavity of LSD1, forms a catalytic chamber where the substrate lysine is oriented and positioned to interact with the | ||
| + | FAD co-factor to initiate demethylation. The specific residues making up the pocket include valine-317, glycine-330, alanine-331, methionine 332, valine-333, phenylalanine-338, leucine-569, asparagine-660, lysine-661, tryptophan 695, serine 749, serine 760, and tyrosine-761. | ||
| + | |||
== Disease == | == Disease == | ||
Revision as of 16:19, 9 April 2019
LSD Demethylase
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
- ↑ Khalid MF, Damha MJ, Shuman S, Schwer B. Structure-function analysis of yeast RNA debranching enzyme (Dbr1), a manganese-dependent phosphodiesterase. Nucleic Acids Res. 2005 Nov 7;33(19):6349-60. doi: 10.1093/nar/gki934. Print, 2005. PMID:16275784 doi:http://dx.doi.org/10.1093/nar/gki934