User:Caitlin Marie Gaich/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
[[Image:Main_bonding_cartoon_3.png|400px|right|thumb|Figure 1]] | [[Image:Main_bonding_cartoon_3.png|400px|right|thumb|Figure 1]] | ||
== Hat1/Hat2 Complex Structure == | == Hat1/Hat2 Complex Structure == | ||
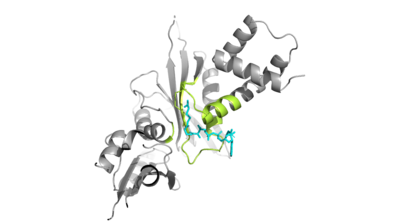
| - | + | Hat1 is not catalytically active until it binds with HAT2 to form the <scene name='81/811718/Hat1_hat2_complex_aco_and_h4/1'>complex</scene>. HAT1 structure includes 317 residues and is identified as <scene name='81/811718/Hat1_protein/1'>chain A</scene>. HAT2 is identified as <scene name='81/811718/Hat2_protein/1'>chain B</scene>, which includes 401 residues. The activated complex acetylates residues in the 38 residue span of <scene name='81/811718/Portion_of_histone_4/1'>Histone 4</scene>. | |
| - | + | ||
| + | The HAT1 and HAT2 interface is stabilized by hydrogen bonds, salt bridges, and hydrophobic interactions. Most of these interactions are located in LP1 of the HAT1 domain, which forms a well-ordered helix. There are three major areas where hydrogen bonds are present. The side chain atoms of <scene name='81/811717/Tyr199_asp308_ala202/3'>Tyr199 and Asp308 </scene> with the main chain nitrogen of Ala202 in HAT1. The side chain of <scene name='81/811717/Lys211phe205_and_leu288arg282/4'>Lys211 and Arg282</scene> makes hydrogen bonds with Leu288 and Phe205 respectively. The last area of hydrogen bonds between HAT1 and HAT is found between <scene name='81/811717/Serine_hydrogen_bonds/2'>Ser263 and Asp 206</scene>. | ||
| - | <scene name='81/811717/Tyr199_asp308_ala202/3'>Tyr199, Asp308, Ala202</scene> | ||
| - | <scene name='81/811717/Lys211phe205_and_leu288arg282/4'>Lys211, Phe205, Leu288, Arg282</scene> | ||
<scene name='81/811717/Hydrophobic_core/2'>Hydrophobic core</scene> | <scene name='81/811717/Hydrophobic_core/2'>Hydrophobic core</scene> | ||
| - | + | ||
Revision as of 22:14, 9 April 2019
Histone Acetyltransferase HAT1/HAT2 Complex, Saccharomyces cerevisiae
| |||||||||||