We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Sean Callahan/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
A unique and defining feature of LSD1 is the 100 residue long insertion between the two parts of the oxidase domain in the primary structure. It is known as the <scene name='81/811712/Tower_domain/2'>Tower Domain</scene> and spans from residues 419-520. This domain is unique, yet vital to LSD1 function. Specifically, it is hypothesized to be a binding platform of LSD1 to the CoRest complex, as well as a site of allosteric regulation. The CoRest complex is a group of proteins responsible for the silencing of neuronal genes in non-neural cells, and the binding of LSD1 to this complex activates its demethylase activity. It is composed to two long alpha helices(TaA and TaB) that extend from the core of the protein. The helices hold each other in place through hydrophobic interactions. The TaB helix is the shorter of the two and is connected to a helix in the oxidase domain (aD). aD is essential for active site formation, and TaB is thought to be responsible for the correct <scene name='81/811711/Tab_and_ad_helix_interaction/1'>positioning</scene> of aD<ref name="Stavropoulos">PMID: 16799558</ref>. | A unique and defining feature of LSD1 is the 100 residue long insertion between the two parts of the oxidase domain in the primary structure. It is known as the <scene name='81/811712/Tower_domain/2'>Tower Domain</scene> and spans from residues 419-520. This domain is unique, yet vital to LSD1 function. Specifically, it is hypothesized to be a binding platform of LSD1 to the CoRest complex, as well as a site of allosteric regulation. The CoRest complex is a group of proteins responsible for the silencing of neuronal genes in non-neural cells, and the binding of LSD1 to this complex activates its demethylase activity. It is composed to two long alpha helices(TaA and TaB) that extend from the core of the protein. The helices hold each other in place through hydrophobic interactions. The TaB helix is the shorter of the two and is connected to a helix in the oxidase domain (aD). aD is essential for active site formation, and TaB is thought to be responsible for the correct <scene name='81/811711/Tab_and_ad_helix_interaction/1'>positioning</scene> of aD<ref name="Stavropoulos">PMID: 16799558</ref>. | ||
| + | ==Regulation== | ||
| + | === Allosteric Stie === | ||
| + | LSD-1 is a protein that can be regulated by outside factors. The tower domain and oxidase domain are connected by a <scene name='81/811711/Regulator_loop/6'>tower oxidase connector</scene>. The oxidase domain is what holds the catalytic chamber for LSD-1. This makes the oxidase domain a very sensitive domain. Any change to the catalytic chamber could drastically reduce the ability for the methylated amine to fit in the binding site correctly. Because the tower domain is attached to the oxidase domain, the tower domain and the connector region become allosteric sites. Any interaction with these two sites is hypothesized to drastically change the enzyme activity. This has further pushed the assumption that the CoRest complex interacts with the tower region.<ref>PMID:16799558</ref> | ||
| + | ===Androgen Receptor=== | ||
| + | LSD-1 will demethylate mono or di-methylated lysines. However it will not demethylate just any lysine. It will only demethylate lysine H3-K4. This factor can be regulated by the androgen receptor. The [http://proteopedia.org/wiki/index.php/Androgen_receptor#Function androgen receptor] is a protein that is involved in DNA transcription. When it interacts with LSD-1 it will no longer demethylate H3-K4, but will now demethylate H3-K9. This attribute allows LSD-1 to work on a wider range of residues.<ref>PMID:16799558</ref> | ||
| + | ==Mechanism== | ||
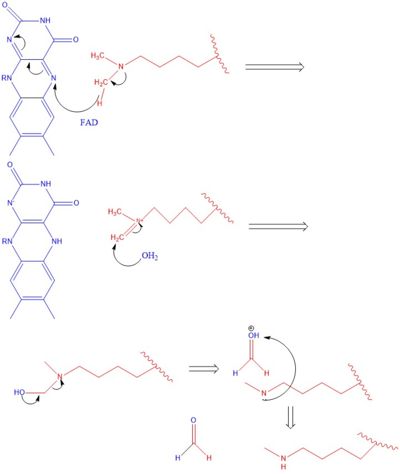
| + | The methylation of lysine via LSD1 occurs through a 2 electron process. Shown in Figure 3 the first step involves the <scene name='81/811710/Fad_highlight/1'>Flavin Adenine Dinucleotide cofactor</scene> initiating a hydride transfer from the one of the two methyl groups bound to the nitrogen at the lysine tail, via a N5 on the flavin group. An imine cation is then formed at the end of the lysine tail to compensate for the loss of hydride. In the next step the imine is hydrolyzed and transitions to an Hemiaminal. This then breaks down easily to formaldehyde and the product lysine. | ||
| + | [[Image:LSD1 Mech.jpg|400px|right|thumb|Figure 3]] | ||
| + | |||
| + | ===Hydrophobic Pocket=== | ||
| + | The hydrophobic pocket located in the active site cavity of LSD1, forms a catalytic chamber where the substrate lysine is oriented and positioned to interact with the | ||
| + | FAD co-factor to initiate demethylation. The specific residues making up the pocket include valine-317, glycine-330, alanine-331, methionine 332, valine-333, phenylalanine-338, leucine-569, asparagine-660, lysine-661, tryptophan 695, serine 749, serine 760, and tyrosine-761. The <scene name='81/811712/Hydrophobic_pocket/1'>hydrophobic pocket</scene> shown in green surrounds the FAD in a way such that it exposes the <scene name='81/811710/Fad_n5/4'>catalytic nitrogen(N5)</scene> that is responsible for the two electron demethylation. Another three separate pockets help bind the histone tail residues to the substrate lysine which is essential for identifying different modifications of the histone tail. | ||
| + | |||
| + | ==Application== | ||
| + | LSD1 plays a pivotal role in many biological processes such as cell growth, epithelial-mesenchymal transition, stem cell biology, malignant transformation of cells, and cell differentiation. Malfunctioning of these activities can lead to life threatening diseases such as acute myeloid leukemia and acute lymphoblastic leukemia. Evidence has shown that these activities have ties to prostate and small cell lung cancer so studies have been done to find a reliable inhibitor for LSD1. Monamine oxidases utilize the FAD co-factor much like LSD1 and inhibitors of monamine oxidase have proven to be successful in inhibiting the activity of LSD1. Thus inhibition of LSD1 opens new pathways for possible treatments for cancers and disorders <ref name="Abdel-Magid">PMID:29152043</ref>. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| + | <ref>PMID:16799558</ref> | ||
<ref name=”Qian">PMID: 16299514 </ref> | <ref name=”Qian">PMID: 16299514 </ref> | ||
<ref name=”Forneris">PMID: 16223729 </ref> | <ref name=”Forneris">PMID: 16223729 </ref> | ||
| Line 28: | Line 44: | ||
<ref name="Da">PMID: 16461455</ref> | <ref name="Da">PMID: 16461455</ref> | ||
<ref name="Stavropoulos">PMID: 16799558</ref> | <ref name="Stavropoulos">PMID: 16799558</ref> | ||
| + | <ref name="Abdel-Magid">PMID:29152043</ref> | ||
<references/> | <references/> | ||
Revision as of 00:39, 12 April 2019
Lysine Specific Demethylase 1 (Homo sapiens)
| |||||||||||
References
- ↑ 1.0 1.1 Forneris F, Binda C, Vanoni MA, Mattevi A, Battaglioli E. Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 2005 Apr 11;579(10):2203-7. doi: 10.1016/j.febslet.2005.03.015. PMID:15811342 doi:http://dx.doi.org/10.1016/j.febslet.2005.03.015
- ↑ Qian C, Zhang Q, Li S, Zeng L, Walsh MJ, Zhou MM. Structure and chromosomal DNA binding of the SWIRM domain. Nat Struct Mol Biol. 2005 Dec;12(12):1078-85. Epub 2005 Nov 20. PMID:16299514 doi:10.1038/nsmb1022
- ↑ 3.0 3.1 Da G, Lenkart J, Zhao K, Shiekhattar R, Cairns BR, Marmorstein R. Structure and function of the SWIRM domain, a conserved protein module found in chromatin regulatory complexes. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2057-62. Epub 2006 Feb 3. PMID:16461455
- ↑ 4.0 4.1 4.2 Stavropoulos P, Blobel G, Hoelz A. Crystal structure and mechanism of human lysine-specific demethylase-1. Nat Struct Mol Biol. 2006 Jul;13(7):626-32. Epub 2006 Jun 25. PMID:16799558 doi:10.1038/nsmb1113
- ↑ Stavropoulos P, Blobel G, Hoelz A. Crystal structure and mechanism of human lysine-specific demethylase-1. Nat Struct Mol Biol. 2006 Jul;13(7):626-32. Epub 2006 Jun 25. PMID:16799558 doi:10.1038/nsmb1113
- ↑ Stavropoulos P, Blobel G, Hoelz A. Crystal structure and mechanism of human lysine-specific demethylase-1. Nat Struct Mol Biol. 2006 Jul;13(7):626-32. Epub 2006 Jun 25. PMID:16799558 doi:10.1038/nsmb1113
- ↑ 7.0 7.1 Abdel-Magid AF. Lysine-Specific Demethylase 1 (LSD1) Inhibitors as Potential Treatment for Different Types of Cancers. ACS Med Chem Lett. 2017 Oct 27;8(11):1134-1135. doi:, 10.1021/acsmedchemlett.7b00426. eCollection 2017 Nov 9. PMID:29152043 doi:http://dx.doi.org/10.1021/acsmedchemlett.7b00426
- ↑ Stavropoulos P, Blobel G, Hoelz A. Crystal structure and mechanism of human lysine-specific demethylase-1. Nat Struct Mol Biol. 2006 Jul;13(7):626-32. Epub 2006 Jun 25. PMID:16799558 doi:10.1038/nsmb1113
- ↑ Qian C, Zhang Q, Li S, Zeng L, Walsh MJ, Zhou MM. Structure and chromosomal DNA binding of the SWIRM domain. Nat Struct Mol Biol. 2005 Dec;12(12):1078-85. Epub 2005 Nov 20. PMID:16299514 doi:10.1038/nsmb1022
- ↑ Forneris F, Binda C, Vanoni MA, Battaglioli E, Mattevi A. Human histone demethylase LSD1 reads the histone code. J Biol Chem. 2005 Dec 16;280(50):41360-5. doi: 10.1074/jbc.M509549200. Epub 2005 , Oct 13. PMID:16223729 doi:http://dx.doi.org/10.1074/jbc.M509549200