The protein we are focusing one is a protein kinase receptor to a family of ligands called angiopoietins. This receptor is a Tyrosine Kinase TIE2. We are going to analyze the of this protein.
It acts as cell-surface receptor for the ligands ANGPT1, ANGPT2 and ANGPT4 and regulates among others angiogenesis, endothelial cell survival and maintenance of vascular quiescence. It is important in the regulation of both normal physiologic and pathologic angiogenesis. The later is a fundamental step in the transition of tumors from a benign state to a malignant one.
Angiogenesis is the process in which new blood vessels are formed from pre-existing blood vessels. The growth of these new blood vessels requires migration and proliferation of endothelial cells (ECs). It is an event controlled by angiogenic growth factors such as vascular endothelial growth factor (VEGF).
While ANGPT1 is a TIE2 agonist and has a higher binding affinity to it than ANGPT2, ANGPT2 can act as a context-dependent agonist. Thus, the ANGPT/TIE2 kinase signaling pathway is an attractive anti-vascular target.
Function
In vascular development, cellular processes are controlled by molecular signal transduction pathways. Those are under the influence of growth factor receptors : the tyrosine kinases present on the surface of endothelial cells. When ligands bind to these receptors, in this case ANGPT1 or ANGPT2, there is oligomerization provoking the activation of the kinase and autophosphorylation.
• Ligands and their binding
TIE2 maintains the vascular integrity of mature vessels by enhancing endothelial barrier function and inhibiting apoptosis of endothelial cells.[1]
ANGPT1 is a TIE2 agonist : in vitro, it binds to TIE2 and induces its activation via tyrosine phosphorylation. In vivo, it was proven that inactivation of ANGPT1 or over expression of ANGPT2 produce similar effects.[2]
ANGPT2 is a competitive antagonist of TIE2 or a partial agonist of TIE2 depending on the context. In stressed ECs, one recent report suggests that ANGPT2 may activate TIE2 signaling in the absence of ANGPT1 and in high concentrations.
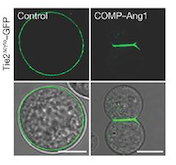
Fig 1. These cells express Tie2 and are marked with GFP. They were incubated with vehicle (control; left column) and COMP–Ang1 (right column). The scale bars represent 10 μm.[2]
• Signal transduction and kinase activity
Receptor tyrosine kinases are transmembrane proteins with a ligand-binding extracellular domain, a single membrane-spanning domain, a juxtamembrane region, a catalytic domain, and a C-terminal tail.[3] In cell culture, ANGPT1 induces phosphorylation of TIE2 and stimulates endothelial cell migration and survival.
The activation of the receptor is due to a ligand-induced dimerization : the extracellular receptor domain dimerization brings the cytosolic kinase domains next to each other for intermolecular autophosphorylation. The latter occurs when one subunit of the dimeric receptor phosphorylates tyrosine residues on the other subunit. It happens in a sequential manner : Tyr-992 in the kinase activation loop is phosphorylated first, followed by autophosphorylation at Tyr-1108 and at additional tyrosine residues. Autophosphorylation also has multiple functions including recruitment of downstream signaling molecules.[3]
Looking more closely at the TIE2 intracellular domain, 1106 is found at the base of a loop formed between the C-terminus tail and the C-terminus lobe of the kinase. The OH group of Tyr-1106 is thus directly into the solvent and accessible to phosphorylation. However, Tyr-1100 is not solvent exposed : thereby implying that the carboxy-terminal tail must undergo a conformational change upon activation of the receptor to expose this tyrosine residue for phosphorylation.[4]
Consequent to ANGPT1 stimulation, the SH2 domain-containing p85 subunit of phosphatidylinositol (PI) 3-kinase is recruited to TIE via tyrosine residue 1100 in the C-end tail of the receptor, leading to activation of the enzyme.[4]
Interestingly, inhibition of PI 3′ kinase activity can only partially inhibit the chemotactic effect of ANGPT1 on endothelial cells, thereby implying that additional TIE2 binding partners may also contribute to ANGPT1-mediated endothelial cell migration. Phosphorylation of TIE2 further results in its association with a docking protein related to downstream of kinase (Dok), known as Dok-R, it allows Dok-R to serve as a substrate of TIE2 and thereby become tyrosine phosphorylated.[4]
Structural highlights
The kinase domain of the TIE2 receptor is composed of two chains : A & B.
• Catalytic activation
ATP + L-tyrosyl-[protein] = ADP + H+ + O-phospho-L-tyrosyl-[protein]
Angiopoietin binding leads to receptor dimerization and activation by autophosphorylation at Tyr-992 on the kinase activation loop.
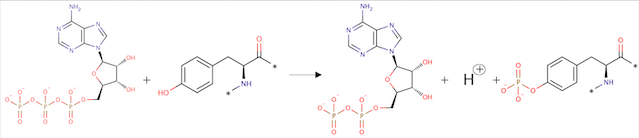
Fig 2. Phosphorylation reaction
• Description of total protein
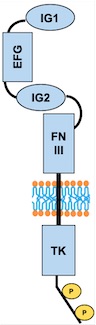
Fig 3. Scheme of the whole TIE2 receptor
Important sites
AA= Amino Acid
– Binding site :
– Active site :
Regions
– Extracellular region : AA 23 to 748 (already found 3D structures: 2gy5, 2gy7, 4k0v, 5mya, 5myb, 5utk)
– Transmembrane region : AA 749 to 769
– Cytoplasmic region : AA 770 to 1124 (already found 3D structures: 1fvr, 2oo8, 2osc, 2wqb, 3bea, 3l8p, 4x3j)
Domains
– AA 44 to 123: Ig-like C2-type 1
– AA 210 to 252: EFG-like 1
– AA 254 to 299: EFG like 2
– AA 301 to 341: EFG-like 3
– AA 350 to 440: Ig-like C2-type 2
– AA 447 to 541: Fibronectin type-III 1 (FN III)
– AA 545 to 636: Fibronectin type-III 2
– AA 641 to 735: Fibronectin type-III 3
– AA 824 to 1096: Protein kinase
• Secondary structure
| Type of secondary structure
| AA position
|
| Beta strand
| 816 - 818
|
| Helix
| 821-823
|
| Beta strand
| 835-831
|
| Helix
| 833-835
|
| Beta strand
| 837-845
|
| Beta strand
| 848-858
|
| Helix
| 864-866
|
| Helix
| 868-876
|
| Beta strand
| 888-894
|
| Beta strand
| 897-902
|
| Helix
| 910-915
|
| Helix
| 919-922
|
| Helix
| 924-929
|
| Beta strand
| 932-936
|
| Helix
| 938-957
|
| Helix
| 967-969
|
| Beta strand
| 970-972
|
| Helix
| 974-976
|
| Beta strand
| 978-980
|
| Beta strand
| 986-989
|
| Turn
| 1002-1004
|
| Helix
| 1007-1012
|
| Helix
| 1017-1032
|
| Turn
| 1038-1041
|
| Helix
| 1044-1050
|
| Helix
| 1051-1053
|
| Helix
| 1065-1074
|
| Helix
| 1079-1081
|
| Helix
| 1085-1097
|
| Beta strand
| 1098-1100
|
| Helix
| 1118-1120
|
• Others amino acid modifications
| Type of modification
| AA position
|
| Phosphotyrosine
| 860
|
| Phosphotyrosine
| 992
|
| Phosphotyrosine
| 1102
|
| Phosphotyrosine
| 1108
|
• Sequence
Due to alternative splicing, several sequences are possible for the protein. There are therefore several isoforms, here we show isoform 1 chosen as the canonical sequence.
The other isoforms differ in missing sequences.
Alternative sequence : Isoform 3: AA 788 missing
Disease
• Dominantly inherited venous malformations (VMCM)
Disease description: A vascular morphogenesis error characterized by dilated serpiginous channels. All mutations occur in the protein kinase domain.
– Mutation in position 849 : Arginine → Tryptophane: Change from large size and basic (R) to large size and aromatic (W). Increased autophosphorylation and kinase activation; no effect on location at membrane.
Arginine at position 849 is found in six residues upstream of the invariant lysine K855 in the kinase domain (sequence preserved among the human, bovine, murine and rat TIE2 sequences). This seems to prove that a basic amino acid is essential for this position. In addition, arginine located a few amino acids before invariant lysine is involved in stabilizing the kinase domain (hydrogen binding of arginine with a proline downstream). It is therefore possible that R849 may also be involved in the stabilization of the kinase domain. Thus, the substitution of R849 by a W could modify the conformation of the kinase domain, leading to a decrease in inhibitory mechanisms and involving autophosphorylation.[5]
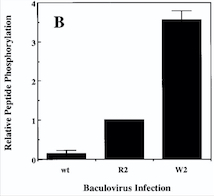
Fig 4. Diagram : Comparison of the Kinase Activities of Normal and Mutant TIE2 Receptors. (B) Cells infected with wild-type baculovirus (wt) or virus expressing normal TIE2 (R2) or mutant TIE2 (W2). Cells expressing the mutation at position 849 (Arginine → Tryptophan) have an autophosphorylation activity 6 to 10 times higher than wild cells.[5]
With this mutation, Venous Malformations (VMs) contain a Disproportionately high ratio of Endothelial Cells (ECs) to Smooth Muscle Cells (SMCs)
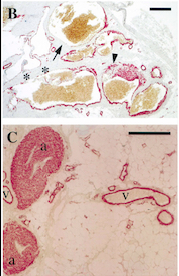
Fig 5. Pictures of immunohistochemistry of VMs with Antibodies against Smooth Muscle Cells 𝛂-Actin [5]
B = Abnormal channels
C = Normal veins (v) and arteries (a)
Scale bars, 200 𝛍m.
Antibodies directed against SMCs 𝛂-Actin from cells with VMs show that the vessels have a specific and abnormal staining (B) compared to normal vessels (C)
– Mutation in position 897 : Tyrosine → Cystéine. Change from large size and aromatic (Y) to medium size and polar (C). An error of vascular morphogenesis characterized by dilated, serpiginous channels
– Mutation in position 897 : Tyrosine → Sérine. Change from large size and aromatic (Y) to small size and polar (S). An error of vascular morphogenesis characterized by dilated, serpiginous channels.
– Mutation in position 915 : Arginine → Histidine. Change from large size and basic (R) to medium size and polar (H). An error of vascular morphogenesis characterized by dilated, serpiginous channels.
– Mutation in position 918 : Arginine → Cystéine. Change from large size and basic (R) to medium size and polar (C). An error of vascular morphogenesis characterized by dilated, serpiginous channels.
– Mutation in position 919 : Valine → Leucine. Similar physico-chemical property. Both residues are medium size and hydrophobic. An error of vascular morphogenesis characterized by dilated, serpiginous channels.
– Mutation in position 925 : Alanine → Sérine. Change from small size and hydrophobic (A) to small size and polar (S). Increased ligand-independent autophosphorylation and kinase activation.
– Mutation in position 1100 : Lysine → Asparagine. Change from large size and basic (K) to medium size and polar (N). Increased ligand-independent autophosphorylation and kinase activation.
•Mutagenesis
– Mutation in position 855 : Loss of kinase activity
– Mutation in position 1102 : Removes interaction with CHS1
Medical relevance
•Venous Malformations
Venous malformations can cause significant morbidity due to pain, disfigurement and organ dysfunction. Before understanding a lot better the mechanisms leading to this disease, therapies were limited to compression therapy and ablation of malformed veins by sclerotherapy and surgery.[6]
A gene test for TIE2 and PIK3CA mutations is the most definite biomarker for VMs. The mutations in the sequence of this proteins cover a large proportion of the causes (about 80%) of all VMs.
In a blood coagulation reaction, fibrinogen is transformed to fibrin that is cleaved by plasmin in fibrinolysis, resulting in the formation of D‐dimers as a fibrin degradation product.
Unlike other vascular malformations, VMs patients often have elevated D‐dimers. D‐dimer testing has shown to be useful to separate VMs from other vascular or lymphatic malformations which usually present with normal D‐dimers. Interestingly, VM patients with identified TIE2 or PIK3CA mutations had high D‐dimers when compared to patients with no detectable mutation in these genes. A high serum level of D‐dimers is not solely due to static blood flow in the lesions, but also to an intrinsic signalling defect in ECs due to constantly high TIE2/PIK3CA activity.[6]
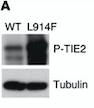
Fig 6. Western blot of p-TIE2 in Human Endothelial Cells transfected with TIE2-WT (Wild type) or with mutant TIE2 (L914F). Tubulin served as loading control. The hyperphosphorylation is clearly visible.[7]
Thus, genetic and transplantation‐based models offer versatile tools to study the pathology of VMs, as well as the efficacy and safety of potential molecular therapies.
Rapamycin is the first molecular therapy for VMs. It is currently being tested in a multicenter clinical trial on lymphatico-vascular malformations.[7]
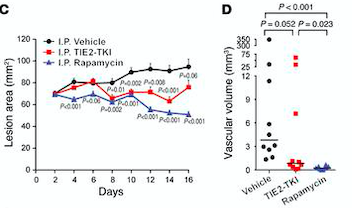
Fig 7. (C) HUVECs lesional area measured every 2 days for 16 days. (D) Vascular volume at day 15 measured by analysis of color Doppler 3D image stacks. When compared with the vehicle-treated group, the lesional area was significantly smaller in the rapamycin-treated group from day 4 to day 16 and in the TIE2-TKI–treated group from day 8 to day 14.[7]
•Cancers
Also in malignant cancers, combinations of pro-angiogenic signals activate endothelial cells attracted to the tumor microenvironment, from there enhancing vascular growth. It has previously been suggested that inhibiting angiogenesis by targeting the regulation and cross-interaction of such signals could form the basis of efforts aimed at engineering cancer therapeutics. Unfortunately, that method has only limited clinical benefit.[8]








