Introduction
The protein PDB 3CFB, or EP2-19G2, is a blue luminescent antibody with diverse applications, such as mercury sensing and DNA labeling. 3CFB was first found in Mus musculus, or the common house mouse, with ties to immune system functions as it has a similar structure, domain, and folding to known immunoglobulins. Both chains of this protein have an immune system-like structure. This protein, a monoclonal antibody, is typically found in complex with a trans-stilbene. It’s luminescent quality makes its presence easy to identify, which makes it useful in biosensing applications. The signature glow is created from an electron transfer reaction from the trans-stilbene to a tryptophan in the antibody. This is unique due to its bluish color and the long duration of an especially bright light, since the light that this protein emits is magnitudes greater than comparable fluorescent compounds. It’s structure includes 864 residues with 4 separate chains, A, B, H, and L.
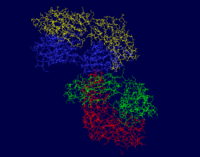
Chains H and L are both sequentially unique to this protein. The A chain is pictured in yellow, the L chain in red, the B chain in blue, and the H chain in green.
Ligand Interactions
Ligands of this protein include glycerol and 4-(4-Styryl-Phenyl Carbamoyl)-Butyric Acid. In the figure, the three smaller molecules are the sites glycerol interaction and the larger molecules the site of 4-(4-Styryl-Phenyl Carbamoyl)-Butyric Acid. Glycerol is represented by GOL and 4-(4-Styryl-Phenyl Carbamoyl)-Butyric Acid is represented by SPB. GOL has significantly more conformations than SPB that can be bound by this protein, which is due to this protein having 3 separate binding sites for it. SPB only has two different conformations, and both are bound in the same binding pocket, although they are attached to two separate chains in the binding pocket. The four binding sites are shown in the linked figures, with example ligands in them. Note that one of the GOL binding sites is extremely close the the SPB binding site. The SPB binding site is on the deep interior of the protein, whereas all of the GOL binding sites are closer to the surface of the protein. GOL is bound by chains A, H, and L. SPB is bound by chains B and L. Specific ligand interactions are represented by SPB 301 L, GOL 402 L, GOL 406 L, GOL 405 H, GOL 401 A, GOL 403 A, GOL 404 A, and SPB 302 A. The first set of three letter is the ligand that is bound, the number represents the specific conformation of the ligand, and the last letter represents the chain being interacted with.
Light and Heavy Chains
The light chains of this protein include the A and L chains. The A chain is made up of 219 residues in total, and is an L polypeptide. The A chain has 3 helices composed of 11 residues, and 25 strands of beta sheets with 113 residues. This strand is 5% helical and 51% beta sheet, which means that 44% of this chain is not a part of a secondary structure. The L chain is also made up of 219 residues total, and is a L polypeptide. The L chain has 3 helices with 12 residues total, and 25 strands of beta sheets made up of 105 residues. This chain is 5% helical and 47% beta sheet, meaning that 48% of this chain is not part of a secondary structure. Chain A has binding sites for GOL A 403, GOL A 404, SPB B 302, and GOL A 401. Chain L has the binding sites for SPB L 301, GOL L 402, and GOL L 406.
The heavy chains of this protein include the B and H chains. It is a total of 426 residues long. Both chains are 213 residues long and are L polypeptides. Chain B is 5% helical and 51% beta sheet, with 44% of its residues not having a secondary structure. Chain H is 5% helical and 50% beta sheet, with 45% of its residues not having a secondary structure. Chain B contains the binding site for SPB B 302, and Chain H has binding sites for both GOL H 405 and SPB L 301.
The photos below shows that the majority of this protein is composed of beta sheets with the occasional alpha helix, however a good portion of this protein is not a part of a secondary structure. In the first figure, the A chain is pictured in yellow, the L chain in red, the B chain in blue, and the H chain in green. In the second figure, alpha helices are pictured in red while beta sheets are shown in yellow. The portions of 3CFB that do not form a secondary structure are shown by white strands.
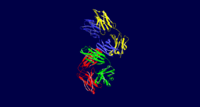

Luminescent Quality
While this protein was originally known to be fluorescent, some important differences led researcher to believe that it’s actual light emitting mechanism was not that of fluorescence. For example, this protein gives off more light at higher temperatures whereas a typical fluorescent dye gives off less light as the temperature increases. Most fluorescent molecules are non-organic, whereas this molecule is a protein and emits a light that is significantly brighter than non-organic fluorescent dyes. Although this protein was previously referred to as a fluorescent protein because of its light generation and its close relation to true fluorescent proteins, it is more properly referred to as luminescent or light emitting. Instead of the glow being caused by an excited electron’s decay into a lower energy state (as it would be in typical fluorescence), it is caused by a charge transfer reaction between a tryptophan residue and the trans-stilbene complex that undergoes deeply inverted electron hole recombination. This protein's rigid matrix allows this recombination to occur, and allows this excited state to endure for a longer time period than is standard for light emitting proteins. Protein luminescence through deeply inverted electron hole recombination is very rare, and 3CFB is a very unique example of this. This electron transfer is the reason the antibody is referred to as luminescent instead of fluorescent, and this reaction can also be elucidated upon UV (ultraviolet) excitation. The luminescence of this protein is increased by two orders of magnitude as compared to other antibody-stilbene complexes. A related protein, PDB 3CFE, does emit a purple fluorescence. The blue color of 3CFB is due to a 30 nm shift in the light spectrum from that of 3CFE. The shift is mostly due to an altered tryptophan residue, which allows this antibody to emit such a bright light. This residue is also responsible for the duration of the blue light, as it gives the protein a luminescent quality instead of a fluorescent one. The protein 3CFE is in fact fluorescent, as it does not have a Trp residue interacting with the stilbene it is attached to. 3CFB also has a stilbene that is positioned very deep into the active site, which would explain its luminescence and why 3CFE does not exhibit the same phenomenon. This finding also shows that the luminescence of 3CFB is unique to itself, and this quality is not shared with related proteins. This protein does exhibit some fluorescence, however it is significantly dimmer than the light produced due to its luminescence. This can be observed at temperatures below 240 degrees Kelvin, as only stilbene fluorescence is seen at temperatures that low. The luminescence of this protein is exhibited at a greater degree at higher temperatures as opposed to lower ones, so the elicitation of this phenomenon should be done at relatively high temperatures as compared to fluorescent phenomena.
Trans-Stilbene Complex Effect on Luminescence
The protein 3CFB is in complex with a trans-stilbene system, with a total of 7 points of contact through antibody residues. The TrpH103 was found to be the residue responsible for the luminescence of the protein, as when it was mutated to Phe the luminescence disappeared. This was compared to the wild type variant as well as a mutated Tyr to Phe residue. Both of these controls continued to exhibit the characteristic blue glow, whereas the mutated Trp residue did not, which suggests that the TrpH103 residue plays a key role in the luminescent quality of 3CFB. In the scene to the right, the protein is shown in complex with the trans stilbene hapten. The TrpH103 residue is higlighted in magenta below.
Modern Implications
The mechanism in which this protein emits light is similar to the mechanism used in modern day LEDs, which eludes to the possibility of a biological LED. While it is unlikely to find a true LED occurring naturally, the existence of this protein suggests that bioluminescent marine organisms could have utilized systems similar to an LED in order to give off light. The existence of this protein also suggests the possibility of genetically engineering organisms to exhibit this sort of luminesence. The duration of the light 3CFB emits makes it incredibly useful in biosensing. It can be used in methods such as ELISA assays as an antibody. After UV light exposure, if the desired ligand to be bound to 3CFB is present, the wells which contain the desired compound would glow. The duration and brightness of this glow means that it is an incredibly sensitive qualitative test. It can also be used in sensing mercury, the evaluation of catalysts, sensing cysteine residues on viral protein coats, and in other biosensor applications. In the future, there is hope of developing similarly luminescent protein-ligand complexes.
References
Castelvecchi, D. (2008). True Blue. Science News, 173(9), 131–132. Retrieved from http://proxy.library.maryville.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=31227332&site=ehost-live
Debler, E. W., Kaufmann, G. F., Meijler, M. M., Heine, A., Mee, J. M., Pljevaljčić, G., … Lerner, R. A. (2008). Deeply Inverted Electron-Hole Recombination in a Luminescent Antibody-Stilbene Complex. Science, 319(5867), 1232–1235.
Debler, E. W., & Wilson, I. A. (2008, March 18). 3CFB. Retrieved April 22, 2019, from https://www.rcsb.org/structure/3CFB




