Blue Luminescent Antibody Derived from House Mouse
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<StructureSection load='3cfb' size='340' side='right' caption='[[3cfb]], [[Resolution|resolution]] 1.60Å' scene=''> | <StructureSection load='3cfb' size='340' side='right' caption='[[3cfb]], [[Resolution|resolution]] 1.60Å' scene=''> | ||
| - | [[Image:chaincolor.png| | + | [[Image:chaincolor.png|500px|right]] |
== Introduction == | == Introduction == | ||
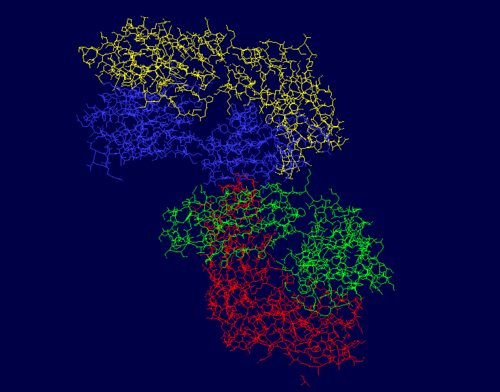
The protein PDB 3CFB, or EP2-19G2, is a blue luminescent antibody with diverse applications, such as mercury sensing and DNA labeling. 3CFB was first found in Mus musculus, or the common house mouse, with ties to immune system functions as it has a similar structure, domain, and folding to known immunoglobulins. Both chains of this protein have an immune system-like structure. This protein, a monoclonal antibody, is typically found in complex with a trans-stilbene. It’s luminescent quality makes its presence easy to identify, which makes it useful in biosensing applications. The signature glow is created from an electron transfer reaction from the trans-stilbene to a tryptophan in the antibody. This is unique due to its bluish color and the long duration of an especially bright light, since the light that this protein emits is magnitudes greater than comparable fluorescent compounds. It’s structure includes 864 residues with 4 separate chains, A, B, H, and L. Chains H and L are both sequentially unique to this protein. The A chain is pictured in yellow, the L chain in red, the B chain in blue, and the H chain in green. | The protein PDB 3CFB, or EP2-19G2, is a blue luminescent antibody with diverse applications, such as mercury sensing and DNA labeling. 3CFB was first found in Mus musculus, or the common house mouse, with ties to immune system functions as it has a similar structure, domain, and folding to known immunoglobulins. Both chains of this protein have an immune system-like structure. This protein, a monoclonal antibody, is typically found in complex with a trans-stilbene. It’s luminescent quality makes its presence easy to identify, which makes it useful in biosensing applications. The signature glow is created from an electron transfer reaction from the trans-stilbene to a tryptophan in the antibody. This is unique due to its bluish color and the long duration of an especially bright light, since the light that this protein emits is magnitudes greater than comparable fluorescent compounds. It’s structure includes 864 residues with 4 separate chains, A, B, H, and L. Chains H and L are both sequentially unique to this protein. The A chain is pictured in yellow, the L chain in red, the B chain in blue, and the H chain in green. | ||
| Line 22: | Line 22: | ||
The heavy chains of this protein include the B and H chains. It is a total of 426 residues long. Both chains are 213 residues long and are L polypeptides. Chain B is 5% helical and 51% beta sheet, with 44% of its residues not having a secondary structure. Chain H is 5% helical and 50% beta sheet, with 45% of its residues not having a secondary structure. Chain B contains the binding site for SPB B 302, and Chain H has binding sites for both GOL H 405 and SPB L 301. | The heavy chains of this protein include the B and H chains. It is a total of 426 residues long. Both chains are 213 residues long and are L polypeptides. Chain B is 5% helical and 51% beta sheet, with 44% of its residues not having a secondary structure. Chain H is 5% helical and 50% beta sheet, with 45% of its residues not having a secondary structure. Chain B contains the binding site for SPB B 302, and Chain H has binding sites for both GOL H 405 and SPB L 301. | ||
| - | The photos | + | The photos to the right show that the majority of this protein is composed of beta sheets with the occasional alpha helix, however a good portion of this protein is not a part of a secondary structure. In the first figure, the A chain is pictured in yellow, the L chain in red, the B chain in blue, and the H chain in green. In the second figure, alpha helices are pictured in red while beta sheets are shown in yellow. The portions of 3CFB that do not form a secondary structure are shown by white strands. |
== Luminescent Quality == | == Luminescent Quality == | ||
| Line 30: | Line 30: | ||
== Trans-Stilbene Complex Effect on Luminescence == | == Trans-Stilbene Complex Effect on Luminescence == | ||
| - | The protein 3CFB is in complex with a trans-stilbene system, with a total of 7 points of contact through antibody residues. The TrpH103 was found to be the residue responsible for the luminescence of the protein, as when it was mutated to Phe the luminescence disappeared. This was compared to the wild type variant as well as a mutated Tyr to Phe residue. Both of these controls continued to exhibit the characteristic blue glow, whereas the mutated Trp residue did not, which suggests that the TrpH103 residue plays a key role in the luminescent quality of 3CFB. In the scene to the right, the protein is shown in complex with the trans stilbene hapten. The TrpH103 residue is higlighted in magenta | + | The protein 3CFB is in complex with a trans-stilbene system, with a total of 7 points of contact through antibody residues. The TrpH103 was found to be the residue responsible for the luminescence of the protein, as when it was mutated to Phe the luminescence disappeared. This was compared to the wild type variant as well as a mutated Tyr to Phe residue. Both of these controls continued to exhibit the characteristic blue glow, whereas the mutated Trp residue did not, which suggests that the TrpH103 residue plays a key role in the luminescent quality of 3CFB. In the scene to the right, the protein is shown in complex with the trans stilbene hapten. The TrpH103 residue is higlighted in magenta in the photo to the right. |
== Modern Implications == | == Modern Implications == | ||
Revision as of 18:35, 24 April 2019
| |||||||||||