We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Madeleine Wilson/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
===The Active Site=== | ===The Active Site=== | ||
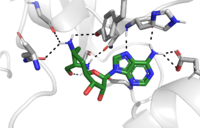
| - | The active site and binding pocket of KMT have several essential characteristics for the overall efficiency. First, the lysine of the histone enters the active site via the lysine access channel comprised of <scene name='81/811092/Tyrosine_channel_2/ | + | The active site and binding pocket of KMT have several essential characteristics for the overall efficiency. First, the lysine of the histone enters the active site via the lysine access channel comprised of <scene name='81/811092/Tyrosine_channel_2/3'>Tyr335 and Tyr337</scene>. Once in the active site, the alkyl part of the histone chain is stabilized by the <scene name='81/811092/Hydrophobic_binding_pocket/1'>hydrophobic binding pocket</scene>, and polar residues are stabilized by hydrogen bonding interactions on the surface. The Tyr335 and Tyr337 are also essential for stabilization of histone chain via hydrogen bonding. |
The <scene name='81/811092/Active_site_w_water/2'>active site</scene> itself contains the cofactor S-adenosyl methionine (SAM) which donates the methyl group in the reaction. <ref name="Xiao" /> | The <scene name='81/811092/Active_site_w_water/2'>active site</scene> itself contains the cofactor S-adenosyl methionine (SAM) which donates the methyl group in the reaction. <ref name="Xiao" /> | ||
[[Image:KMT_mechanism.png|200px|left|thumb|KMT Mechanism]] | [[Image:KMT_mechanism.png|200px|left|thumb|KMT Mechanism]] | ||
Revision as of 19:52, 24 April 2019
Histone Lysine Methyltransferase: Gene Activator
| |||||||||||
References
- ↑ DesJarlais R, Tummino PJ. Role of Histone-Modifying Enzymes and Their Complexes in Regulation of Chromatin Biology. Biochemistry. 2016 Mar 22;55(11):1584-99. doi: 10.1021/acs.biochem.5b01210. Epub , 2016 Jan 26. PMID:26745824 doi:http://dx.doi.org/10.1021/acs.biochem.5b01210
- ↑ 2.0 2.1 doi: https://dx.doi.org/10.1016/j.apsb.2013.04.007
- ↑ 3.0 3.1 Dong X, Weng Z. The correlation between histone modifications and gene expression. Epigenomics. 2013 Apr;5(2):113-6. doi: 10.2217/epi.13.13. PMID:23566087 doi:http://dx.doi.org/10.2217/epi.13.13
- ↑ 4.0 4.1 Del Rizzo PA, Trievel RC. Substrate and product specificities of SET domain methyltransferases. Epigenetics. 2011 Sep 1;6(9):1059-67. doi: 10.4161/epi.6.9.16069. Epub 2011 Sep, 1. PMID:21847010 doi:http://dx.doi.org/10.4161/epi.6.9.16069
- ↑ 5.0 5.1 5.2 5.3 Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003 Feb 6;421(6923):652-6. Epub 2003 Jan 22. PMID:12540855 doi:10.1038/nature01378
- ↑ Schluckebier G, Kozak M, Bleimling N, Weinhold E, Saenger W. Differential binding of S-adenosylmethionine S-adenosylhomocysteine and Sinefungin to the adenine-specific DNA methyltransferase M.TaqI. J Mol Biol. 1997 Jan 10;265(1):56-67. PMID:8995524 doi:http://dx.doi.org/10.1006/jmbi.1996.0711
- ↑ Tamura R, Doi S, Nakashima A, Sasaki K, Maeda K, Ueno T, Masaki T. Inhibition of the H3K4 methyltransferase SET7/9 ameliorates peritoneal fibrosis. PLoS One. 2018 May 3;13(5):e0196844. doi: 10.1371/journal.pone.0196844., eCollection 2018. PMID:29723250 doi:http://dx.doi.org/10.1371/journal.pone.0196844
Student Contributors
Lauryn Padgett, Alexandra Pentala, Madeleine Wilson