Carboxypeptidase
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
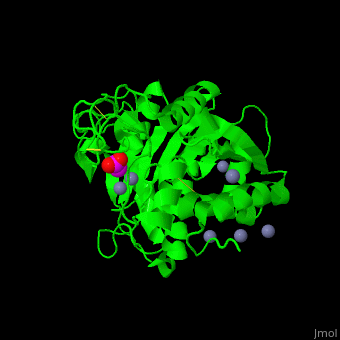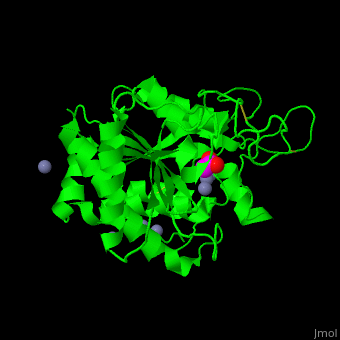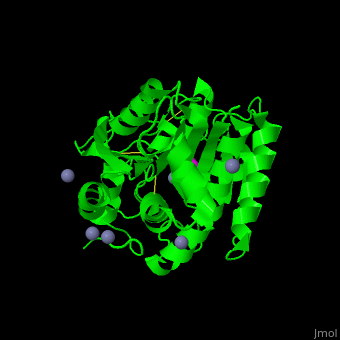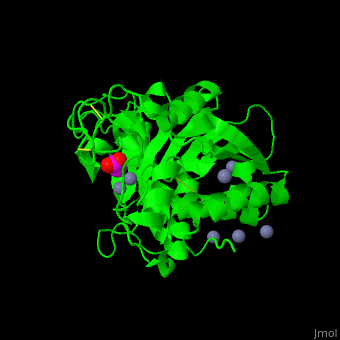
The <scene name='47/478539/Cv/4'>active site Zn+2</scene> is located at the bottom of a pocket open to the surface of the enzyme.<ref>PMID:24010887</ref> | The <scene name='47/478539/Cv/4'>active site Zn+2</scene> is located at the bottom of a pocket open to the surface of the enzyme.<ref>PMID:24010887</ref> | ||
| + | |||
| + | == 3D Structures of Carboxypeptidase == | ||
| + | [[Carboxypeptidase 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
| Line 61: | Line 64: | ||
**[[2pcu]] – hCPA4 + peptide <br /> | **[[2pcu]] – hCPA4 + peptide <br /> | ||
**[[4uee]], [[4uef]], [[4uez]] – hCPA1 + peptide inhibitor<br /> | **[[4uee]], [[4uef]], [[4uez]] – hCPA1 + peptide inhibitor<br /> | ||
| - | **[[4uf4]], [[5om9]] – hCPA1 + inhibitor<br /> | + | **[[4uf4]], [[5om9]], [[6i6z]] – hCPA1 + inhibitor<br /> |
**[[4oxd]] – SpCP + peptidoglycan<br /> | **[[4oxd]] – SpCP + peptidoglycan<br /> | ||
| Line 91: | Line 94: | ||
**[[3glj]] – pCPB residues 16-416 - pig<br /> | **[[3glj]] – pCPB residues 16-416 - pig<br /> | ||
**[[5jc6]] – pCPB catalytic domain residues 111-416 <br /> | **[[5jc6]] – pCPB catalytic domain residues 111-416 <br /> | ||
| - | **[[3wab]], [[3wc5]], [[3wc6]], [[3wc7]], [[5lyf]], [[5lyi]], [[5lyl]], [[4z65]], [[4uia]], [[2piy]], [[2piz]], [[2pj0]], [[2pj1]], [[2pj2]], [[2pj3]], [[2pj4]], [[2pj5]], [[2pj6]], [[2pj7]], [[2pj8]], [[2pj9]], [[2pja]], [[2pjb]], [[2pjc]] – pCPB catalytic domain + inhibitor<br /> | + | **[[3wab]], [[3wc5]], [[3wc6]], [[3wc7]], [[5lyf]], [[5lyi]], [[5lyl]], [[4z65]], [[4uia]], [[2piy]], [[2piz]], [[2pj0]], [[2pj1]], [[2pj2]], [[2pj3]], [[2pj4]], [[2pj5]], [[2pj6]], [[2pj7]], [[2pj8]], [[2pj9]], [[2pja]], [[2pjb]], [[2pjc]], [[5j1q]], [[5zeq]] – pCPB catalytic domain + inhibitor<br /> |
**[[5lyd]], [[4uib]] – pCPB catalytic domain (mutant) + inhibitor<br /> | **[[5lyd]], [[4uib]] – pCPB catalytic domain (mutant) + inhibitor<br /> | ||
**[[5lrg]], [[5lrj]], [[5lrk]] – pCPB catalytic domain + anabaenopepsin peptide<br /> | **[[5lrg]], [[5lrj]], [[5lrk]] – pCPB catalytic domain + anabaenopepsin peptide<br /> | ||
| Line 155: | Line 158: | ||
**[[1obr]], [[3qnv]] – TvCPT – ''Thermoaclinomyces vulgaris''<br /> | **[[1obr]], [[3qnv]] – TvCPT – ''Thermoaclinomyces vulgaris''<br /> | ||
**[[3prt]], [[3v38]], [[4ihm]] – TvCPT (mutant)<br /> | **[[3prt]], [[3v38]], [[4ihm]] – TvCPT (mutant)<br /> | ||
| - | **[[4djl]] – TvCPT + sulfamoyl | + | **[[4djl]], [[5myh]], [[6go2]] – TvCPT + sulfamoyl derivative<br /> |
| - | **[[4iav]] | + | **[[4iav]], [[6q4l]], [[6f79]], [[6f75]], [[6f6q]] – TvCPT (mutant) + sulfamoyl derivative<br /> |
| - | + | ||
**[[4duk]] – TvCPT + benzyl succinic acid<br /> | **[[4duk]] – TvCPT + benzyl succinic acid<br /> | ||
**[[4f8z]] – TvCPT + leucine derivative<br /> | **[[4f8z]] – TvCPT + leucine derivative<br /> | ||
| Line 190: | Line 192: | ||
**[[1w79]], [[1w8q]] – AACP – ''Actinomadura''<br /> | **[[1w79]], [[1w8q]] – AACP – ''Actinomadura''<br /> | ||
| - | **[[3pte]], [[1skf]] – StAACP – ''Streptomyces''<br /> | ||
**[[4rye]] – MtAACP <br /> | **[[4rye]] – MtAACP <br /> | ||
**[[5fsr]] – EcAACP <br /> | **[[5fsr]] – EcAACP <br /> | ||
| + | **[[3pte]], [[1skf]] – StAACP – ''Streptomyces''<br /> | ||
**[[1es2]], [[1es3]], [[1es4]], [[1es5]], [[1esi]], [[1j9m]] – StAACP (mutant) <br /> | **[[1es2]], [[1es3]], [[1es4]], [[1es5]], [[1esi]], [[1j9m]] – StAACP (mutant) <br /> | ||
**[[1lbu]] – SaAACP + Zn – ''Streptomyces albus''<br /> | **[[1lbu]] – SaAACP + Zn – ''Streptomyces albus''<br /> | ||
| + | **[[6c39]] – AACP + Zn – ''Staphylococcus aureus''<br /> | ||
| + | **[[5zhf]] – EfAACP + Zn – ''Enterococcus faecalis''<br /> | ||
| + | **[[5hnm]] – EfAACP (mutant) + Zn <br /> | ||
| + | **[[6a6a]] – EfAACP + Zn + Ala <br /> | ||
| + | **[[5zhw]] – EfAACP + Zn + Ala-Ala<br /> | ||
**[[2wke]], [[1w8y]], [[2vgj]], [[2vgk]], [[4ben]] - AcAACP + antibiotic<br /> | **[[2wke]], [[1w8y]], [[2vgj]], [[2vgk]], [[4ben]] - AcAACP + antibiotic<br /> | ||
**[[1cef]], [[1ceg]], [[1hvb]], [[1pw1]], [[1pw8]], [[1pwc]], [[1pwd]], **[[1pwg]] – StAACP + antibiotic<br /> | **[[1cef]], [[1ceg]], [[1hvb]], [[1pw1]], [[1pw8]], [[1pwc]], [[1pwd]], **[[1pwg]] – StAACP + antibiotic<br /> | ||
| Line 204: | Line 211: | ||
**[[3zcz]] – AcAACP + inhibitor<br /> | **[[3zcz]] – AcAACP + inhibitor<br /> | ||
**[[5j8x]] – EcAACP + inhibitor<br /> | **[[5j8x]] – EcAACP + inhibitor<br /> | ||
| + | **[[6ntz]] – EcAACP + meropenem<br /> | ||
*β-lactamase/D-alanine carboxypeptidase | *β-lactamase/D-alanine carboxypeptidase | ||
| Line 213: | Line 221: | ||
**[[1z8l]], [[2oot]] – hECP II<br /> | **[[1z8l]], [[2oot]] – hECP II<br /> | ||
| - | **[[2c6c]], [[2jbj]], [[2jbk]], [[2or4]], [[3d7d]], [[3d7f]], [[3d7g]], [[3d7h]], [[3iww]], [[3sjf]], [[4jyw]], [[4jz0]], [[4lqg]], [[5ely]], [[5d29]], [[4x3r]], [[4w9y]] – hECP II + inhibitor<br /> | + | **[[2c6c]], [[2jbj]], [[2jbk]], [[2or4]], [[3d7d]], [[3d7f]], [[3d7g]], [[3d7h]], [[3iww]], [[3sjf]], [[4jyw]], [[4jz0]], [[4lqg]], [[5ely]], [[5d29]], [[4x3r]], [[4w9y]], [[6hkz]], [[6hkj]], [[6h7z]], [[6h7y]], [[6f5l]], [[6ety]] – hECP II + inhibitor<br /> |
| + | **[[6fe5]], [[6ez9]], [[5of0]] - hECP II (mutant) + inhibitor<br /> | ||
**[[4p44]], [[4p45]], [[4p4e]], [[4p4d]], [[4p4f]], [[4p4i]], [[4p4j]], [[4p4b]] – hECP II + phosphoramidate inhibitor<br /> | **[[4p44]], [[4p45]], [[4p4e]], [[4p4d]], [[4p4f]], [[4p4i]], [[4p4j]], [[4p4b]] – hECP II + phosphoramidate inhibitor<br /> | ||
| - | **[[4ngm]], [[4ngn]], [[4ngp]], [[4ngq]], [[4ngr]], [[4ngs]], [[4ngt]], [[4oc0]], [[4oc1]], [[4oc2]], [[4oc3]], [[4oc4]], [[4oc5]], [[4ome]] - hECP II + urea derivative<br /> | + | **[[4ngm]], [[4ngn]], [[4ngp]], [[4ngq]], [[4ngr]], [[4ngs]], [[4ngt]], [[4oc0]], [[4oc1]], [[4oc2]], [[4oc3]], [[4oc4]], [[4oc5]], [[4ome]], [[5o5u]], [[5o5t]], [[5o5r]] - hECP II + urea derivative<br /> |
**[[2c6g]] - hECP II + glutamate<br /> | **[[2c6g]] - hECP II + glutamate<br /> | ||
**[[4mcr]], [[4mcs]] - hECP II (mutant) + glutamate<br /> | **[[4mcr]], [[4mcs]] - hECP II (mutant) + glutamate<br /> | ||
Revision as of 08:01, 25 April 2019
| |||||||||||
3D Structures of Carboxypeptidase
Updated on 25-April-2019
See Angiotensin-Converting Enzyme
References
- ↑ Fernandez D, Boix E, Pallares I, Aviles FX, Vendrell J. Structural and Functional Analysis of the Complex between Citrate and the Zinc Peptidase Carboxypeptidase A. Enzyme Res. 2011;2011:128676. doi: 10.4061/2011/128676. Epub 2011 Jul 25. PMID:21804935 doi:10.4061/2011/128676
- ↑ Adler M, Bryant J, Buckman B, Islam I, Larsen B, Finster S, Kent L, May K, Mohan R, Yuan S, Whitlow M. Crystal structures of potent thiol-based inhibitors bound to carboxypeptidase B. Biochemistry. 2005 Jul 5;44(26):9339-47. PMID:15982000 doi:http://dx.doi.org/10.1021/bi0501941
- ↑ Yoshimoto N, Itoh T, Inaba Y, Ishii H, Yamamoto K. Structural Basis for Inhibition of Carboxypeptidase B by Selenium-Containing Inhibitor: Selenium Coordinates to Zinc in Enzyme. J Med Chem. 2013 Sep 24. PMID:24010887 doi:10.1021/jm400816v
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky