We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Lauryn Padgett/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | =Histone Lysine Methyltransferase: Gene Activator= | |
| - | <StructureSection load='1o9s' size='350' frame='true' side='right' caption=' | + | <StructureSection load='1o9s' size='350' frame='true' side='right' caption='Lysine Methyl Transferase' scene='81/811092/Kmt_full/2'> |
| + | == Introduction == | ||
| - | == | + | ===Histone Methylation=== |
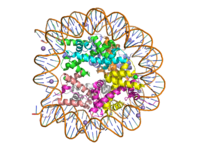
| + | [[Image:human_nucleosome_ray_trace.png|200 px| right| thumb|Human nucleosome particle, pbd code: 5y0c]] | ||
| + | [https://en.wikipedia.org/wiki/Histone Histone proteins] aid in the packing of DNA for the purpose of compacting the genome in the nucleus of the cell and regulating physical accessibility of genes for transcription. The protein itself is an octamer of core proteins H2a, H2b, H3, and H4, which organize into two heterodimers; H1 and H5 act as linker proteins. About 145-157 base pairs wind around a histone heterodimer core. <ref name="DesJarlais">PMID: 26745824</ref> Modifications to histone core proteins can affect the accessibility of transcription factors in the genome, either promoting or inhibiting transcription. Some of these modifications include methylation/demethylation, acetylation/deacetylation, and ubiquitination/deubiquitination. <ref name="Lun">DOI: 10.1016/j.apsb.2013.04.007</ref> | ||
| - | = | + | Specifically, histone methylation is associated with gene activation. <ref name="Dong">PMID: 23566087</ref> Many domain families fall under the histone methylase family, one of these enzymes being the <scene name='81/811092/Set7_domain/2'>SET7 domain</scene> family, which can target H3, H4, or H2a. Sites known for gene activation are Lysine-4, Lys-36, and Lys-79 on H3; whereas, methylation at Lys-9 and Lys- 27 on H3 and Lys-20 on H4 are known for gene inactivation.<ref name="Rizzo">PMID: 21847010</ref> Typically, methylation of some of these sites are always present on both active and inactive genes, extra methylations required for activity; specifics of this characteristic depend on site and species of organism. <ref name="Xiao">doi:10.1038/nature01378</ref> Some tumor related genes such as p53 are site specifically methylated to promote biological function <ref name = "Rizzo" />, whereas hypomethylation of CpG is linked to tumor genesis. <ref name="Lun" /> A particular enzyme in the SET7 domain family is lysine methyltransferase, which acts on the histone by adding a methyl group to Lys4 on H3; the addition results in promotion of gene unwinding and gene transcription. <ref name="Xiao" />, <ref name="Dong" /> |
| - | + | ||
| + | ==Lysine Methyltransferase (KMT) Structure== | ||
| + | ===Overall Secondary Structure=== | ||
| + | Due to the composition of its [https://en.wikipedia.org/wiki/Protein_secondary_structure secondary structure], KMT is an alpha-beta protein . The helical composition includes 3 <scene name='81/811086/Alpha_helices/4'>alpha helices</scene>, with two residing in the SET domain and one in the C-terminal domain. The alpha helices in the SET domain are two turns while the C-terminal helix is by far the largest with 4 turns. There are also 2 <scene name='81/811086/3-10_helices/4'>3-10 helices</scene> in the SET domain which are each one turn. There are 21 total <scene name='81/811086/Beta_sheets/3'>beta strands</scene> which reside in the N-terminal domain and the SET domain. The beta strands are primarily anti-parallel and multiple antiparallel strands are connected by <scene name='81/811086/Type_i_beta_turns/3'>Type I</scene> and <scene name='81/811086/Type_ii_beta_turns/2'>Type II</scene> beta turns. | ||
| + | ===The Active Site=== | ||
| + | The active site and binding pocket of KMT contain residues and shape that ensure both catalytic capability as well as optimal stability. First, the lysine of the histone enters the active site via the <scene name='81/811092/Tyrosine_channel_2/3'>Lysine access channel</scene> comprised of Tyr335 and Tyr337. Feeding the histone into the active site is initially difficult; however, the hydrophobicity of the aromatic rings and slight polarity of the alcohol group on the Tyr side chain are essential for facilitating this process. Once in the active site, the alkyl part of the histone chain is stabilized by the <scene name='81/811092/Hydrophobic_binding_pocket/1'>hydrophobic binding pocket</scene>, and polar residues are stabilized by hydrogen bonding interactions on the surface. The Tyr335 and Tyr337 are also essential for stabilization of histone chain via hydrogen bonding. | ||
| + | The <scene name='81/811092/Active_site_w_water/3'>active site</scene> itself contains the cofactor [https://en.wikipedia.org/wiki/S-Adenosyl_methionine S-adenosyl methionine (SAM)] which donates the methyl group in the reaction. <ref name="Xiao" /> In the active site scene, the structures depict the post-reaction result, where the Lys has been methylated and SAM has been converted to S-adenosyl homocysteine (SAH). | ||
| + | [[Image:KMT_Mechanism_jpg.jpeg|200px|left|thumb|Figure 2. KMT Mechanism]] | ||
| + | The reaction is catalyzed by Tyr305, Tyr245, carbonyl oxygens of the main chain in residues Ala295 and Ser290. Tyr305 and the carbonyl oxygens stabilize and pull electron density off a water to pull on one of the hydrogens off the nitrogen of the lysine, while oxygen of Tyr245 pulls on the other hydrogen of the nitrogen. Both of these actions allow nitrogen to become more nucleophilic and attack the carbon of the methyl group on the SAM, which is attached to a positively charged sulfur. The methyl group is then transferred and the sulfur is neutral; SAM has been converted to (SAH). <ref name="Xiao" /> | ||
| + | ===The C-Terminal Domain=== | ||
| + | The <scene name='81/811091/C_terminal_domain/1'>C-terminal segment</scene> of lysine methyltransferase is essential for the catalytic activity of the enzyme. Hydrophobic packing of the C-terminal segment (residues 345-366) forms the lysine access channel. Residues 337-349 create a <scene name='81/811086/Beta_hairpin/2'>beta hairpin structure</scene> that stabilizes the orientation of two tyrosine residues Tyr 335 and Tyr337 that form the lysine access channel. The hydrophobic packing of the C-terminal <scene name='81/811091/C_terminal_domain/7'>alpha helix</scene> against beta sheet 19 (specifically residue 299) orient the SAM cofactor so the methyl donating group is oriented toward the lysine access channel. donating group is oriented toward the lysine access channel. | ||
| - | The | + | The C-terminal domain of lysine methyltransferase is very important for the catalytic activity of the enzyme. The structures of the <scene name='81/811091/C_terminal_domain/1'>C-terminal domain (residues 345-366)</scene> serve the role of stabilizing the structures in the <scene name='81/811091/C_terminal_domain/16'>SET7 domain (residues 193-344)</scene> in the correct orientation for a reaction in the active site.<ref name="Xiao" /> Hydrophobic interactions in the C-terminal domain are mainly responsible for stabilizing the access channel for the lysine methylation site on histone H3. Residues 337-349 create a beta-hairpin structure that stabilizes the orientation of two tyrosine residues, Tyr335 and Tyr337, that form the opening to the lysine access channel. Furthermore, the hydrophobic packing of alpha-helix 3 against beta-sheet 19, specifically <scene name='81/811091/C_terminal_domain/13'>residues Leu357 and Phe 299</scene>, stabilize the orientation of the <scene name='81/811091/C_terminal_domain/14'>SAM cofactor</scene> so that its methyl donating group is oriented toward the lysine access channel. The orientation of the SAM cofactor is further stabilized with its hydrophobic interactions with C-terminal domain residues <scene name='81/811091/C_terminal_domain/18'>Trp352 and Glu356</scene>. <ref name="Xiao" /> |
| - | <scene name='81/ | + | |
| - | <scene name='81/ | + | |
| - | < | + | |
| - | <scene name='81/ | + | |
| - | <scene name='81/ | + | |
| - | <scene name='81/ | + | |
| - | < | + | |
| + | ===The N-terminal Domain=== | ||
| + | [[Image:Clustal.png|200px|right|thumb|Figure 1. Clustal alignment of N-terminals]] | ||
| + | The N-terminal domain has no notable function in regard to the activity of KMT; in addition to this, no recent studies have been conducted to discover what the exact function of this region is. As this is a highly conserved region across species, it may be hypothesized this domain plays an integral part in stability. | ||
==Inhibitors== | ==Inhibitors== | ||
Revision as of 01:39, 26 April 2019
Histone Lysine Methyltransferase: Gene Activator
| |||||||||||
References
- ↑ DesJarlais R, Tummino PJ. Role of Histone-Modifying Enzymes and Their Complexes in Regulation of Chromatin Biology. Biochemistry. 2016 Mar 22;55(11):1584-99. doi: 10.1021/acs.biochem.5b01210. Epub , 2016 Jan 26. PMID:26745824 doi:http://dx.doi.org/10.1021/acs.biochem.5b01210
- ↑ 2.0 2.1 doi: https://dx.doi.org/10.1016/j.apsb.2013.04.007
- ↑ 3.0 3.1 Dong X, Weng Z. The correlation between histone modifications and gene expression. Epigenomics. 2013 Apr;5(2):113-6. doi: 10.2217/epi.13.13. PMID:23566087 doi:http://dx.doi.org/10.2217/epi.13.13
- ↑ 4.0 4.1 Del Rizzo PA, Trievel RC. Substrate and product specificities of SET domain methyltransferases. Epigenetics. 2011 Sep 1;6(9):1059-67. doi: 10.4161/epi.6.9.16069. Epub 2011 Sep, 1. PMID:21847010 doi:http://dx.doi.org/10.4161/epi.6.9.16069
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003 Feb 6;421(6923):652-6. Epub 2003 Jan 22. PMID:12540855 doi:10.1038/nature01378
- ↑ Schluckebier G, Kozak M, Bleimling N, Weinhold E, Saenger W. Differential binding of S-adenosylmethionine S-adenosylhomocysteine and Sinefungin to the adenine-specific DNA methyltransferase M.TaqI. J Mol Biol. 1997 Jan 10;265(1):56-67. PMID:8995524 doi:http://dx.doi.org/10.1006/jmbi.1996.0711
- ↑ Tamura R, Doi S, Nakashima A, Sasaki K, Maeda K, Ueno T, Masaki T. Inhibition of the H3K4 methyltransferase SET7/9 ameliorates peritoneal fibrosis. PLoS One. 2018 May 3;13(5):e0196844. doi: 10.1371/journal.pone.0196844., eCollection 2018. PMID:29723250 doi:http://dx.doi.org/10.1371/journal.pone.0196844
- ↑ Takemoto Y, Ito A, Niwa H, Okamura M, Fujiwara T, Hirano T, Handa N, Umehara T, Sonoda T, Ogawa K, Tariq M, Nishino N, Dan S, Kagechika H, Yamori T, Yokoyama S, Yoshida M. Identification of Cyproheptadine as an Inhibitor of SET Domain Containing Lysine Methyltransferase 7/9 (Set7/9) That Regulates Estrogen-Dependent Transcription. J Med Chem. 2016 Apr 28;59(8):3650-60. doi: 10.1021/acs.jmedchem.5b01732. Epub, 2016 Apr 18. PMID:27088648 doi:http://dx.doi.org/10.1021/acs.jmedchem.5b01732