User:Asif Hossain/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Introduction == | == Introduction == | ||
| - | Histone deacetylase 8 (HDAC8) is an enzyme that plays a role in controlling gene expression. Specifically, HDAC8 removes an acetyl group off of the ε-amino-Lys 382 of Histone 4's N-terminal core.<ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> [https://en.wikipedia.org/wiki/Histone Histones] consist of eight monomers to form an octomer complex. Each histone has a positive charge which allows interaction with negatively-charged DNA. This prevents transcription factors from accessing DNA, thus, decreasing gene expression. [https://en.wikipedia.org/wiki/Chromatin_remodeling Chromatin remodeling] by | + | Histone deacetylase 8 (HDAC8) is an enzyme that plays a role in controlling gene expression. Specifically, HDAC8 removes an acetyl group off of the ε-amino-Lys 382 of Histone 4's N-terminal core.<ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> [https://en.wikipedia.org/wiki/Histone Histones] consist of eight monomers to form an octomer complex. Each histone has a positive charge which allows interaction with negatively-charged DNA. This prevents transcription factors from accessing DNA, thus, decreasing gene expression. [https://en.wikipedia.org/wiki/Chromatin_remodeling Chromatin remodeling] by histone acetylation and/or deacetylation is an example of [https://en.wikipedia.org/wiki/Epigenetics epigenetic regulation]. [https://en.wikipedia.org/wiki/Histone_acetyltransferase Histone Acetylase 1] (HAT1) catalyzes the addition of an acetyl group onto a histone. The lack of charge on the acetyl group weakens the interaction between DNA and histones which allows transcription factors to access the DNA to increase gene expression. HDAC8 reverses this reaction by catalyzing the removal of these acetyl groups from the Lys to reclaim the positive charge of the histone. This allows the histone to interact with the negative charge on the DNA. As a result, DNA binds more tightly to the histone protein, repressing transcription and gene expression. |
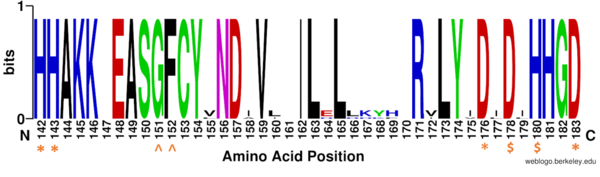
==HDAC Enzymes and Homology== | ==HDAC Enzymes and Homology== | ||
| Line 13: | Line 13: | ||
==HDAC8 Structure== | ==HDAC8 Structure== | ||
| - | The crystal structure of human HDAC8 was determined using x-ray crystallography at a 2.0Å resolution. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The structure includes two structural K ion and one catalytic Zn ion. HDAC8 is bound to a [https://en.wikipedia.org/wiki/P53 p-53] derived diacetylated peptide substrate as opposed to the natural histone substrate. This peptide includes a fluorescent coumarin ring likely used in past kinetic assays. | + | The crystal structure of human HDAC8 was determined using x-ray crystallography at a 2.0Å resolution. <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> The structure includes two structural K ion and one catalytic Zn ion. HDAC8 is bound to a [https://en.wikipedia.org/wiki/P53 p-53] derived diacetylated peptide substrate as opposed to the natural histone substrate. This peptide includes a fluorescent coumarin ring likely used in past kinetic assays. The HDAC8 is made up of a <scene name='81/811084/Beta_sheets/6'>β-sheet</scene> with eight parallel β-strands located between 13 <scene name='81/811084/Alpha_helicesv2/4'>α-helices</scene>. The HDAC8 consists of 377 amino acids. <ref name="Somoza"> Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012 </ref> |
| - | + | ||
| - | The HDAC8 is made up of a | + | |
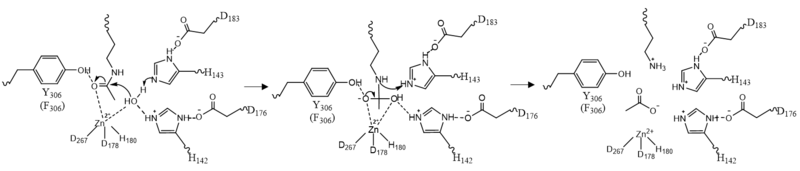
===Zinc Ion=== | ===Zinc Ion=== | ||
Revision as of 13:41, 26 April 2019
Histone Deacetylase 8 (HDAC 8)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047
- ↑ DesJarlais, R., & Tummino, P. J. (2016). Role of histone-modifying enzymes and their complexes in regulation of chromatin biology. Biochemistry, 55(11), 1584-1599. https://doi.org/10.1021/acs.biochem.5b01210
- ↑ 3.0 3.1 3.2 3.3 3.4 Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012
- ↑ Whitehead, L., Dobler, M. R., Radetich, B., Zhu, Y., Atadja, P. W., Claiborne, T., ... & Shao, W. (2011). Human HDAC isoform selectivity achieved via exploitation of the acetate release channel with structurally unique small molecule inhibitors. Bioorganic & medicinal chemistry, 19(15), 4626-4634. https://doi.org/10.1016/j.bmc.2011.06.030
- ↑ 5.0 5.1 5.2 Vannini, A., Volpari, C., Filocamo, G., Casavola, E. C., Brunetti, M., Renzoni, D., ... & Steinkühler, C. (2004). Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proceedings of the National Academy of Sciences, 101(42), 15064-15069. https://dx.doi.org/10.1073%2Fpnas.0404603101
- ↑ Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology, 6(4), a018713. https://doi.org/10.1101/cshperspect.a018713
- ↑ Eckschlager T, Plch, J, Stiborova M, Hrabeta J.Histone deacetylase inhibitors as anticancer drugs. International journal of molecular sciences, 18(7), 1414. 2017. https://dx.doi.org/10.3390%2Fijms18071414