User:Asif Hossain/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
There are four major classes of HDAC proteins (I,II, III, and IV). Other than the Class III “[https://en.wikipedia.org/wiki/Sirtuin sirtuins]” that utilize a [https://pubs.acs.org/appl/literatum/publisher/achs/journals/content/bichaw/2016/bichaw.2016.55.issue-11/acs.biochem.5b01210/20160316/images/medium/bi-2015-01210h_0006.gif NAD<sup>+</sup> cofactor-dependent mechanism], all other HDAC classes use Zn<sup>2+</sup>-assisted catalysis through mechanisms (Figure 3) reminiscent of a typical [https://en.wikipedia.org/wiki/Serine_protease serine protease].<ref name="DesJarlais, R., & Tummino, P. J.">DesJarlais, R., & Tummino, P. J. (2016). Role of histone-modifying enzymes and their complexes in regulation of chromatin biology. Biochemistry, 55(11), 1584-1599. https://doi.org/10.1021/acs.biochem.5b01210 </ref> While Classes I, II, and IV do have some major distinctions such as size of the protein, in general, they share homology at the catalytic site. HDAC 8 is classified as a Class I HDAC alongside HDACs 1-3. In fact, within Class I HDACs, there are many invariant residues involved in the catalytic site (such as His-Asp dyads), Zn-binding, and ligand binding pocket (such as Asp101) (Figure 1). <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> | There are four major classes of HDAC proteins (I,II, III, and IV). Other than the Class III “[https://en.wikipedia.org/wiki/Sirtuin sirtuins]” that utilize a [https://pubs.acs.org/appl/literatum/publisher/achs/journals/content/bichaw/2016/bichaw.2016.55.issue-11/acs.biochem.5b01210/20160316/images/medium/bi-2015-01210h_0006.gif NAD<sup>+</sup> cofactor-dependent mechanism], all other HDAC classes use Zn<sup>2+</sup>-assisted catalysis through mechanisms (Figure 3) reminiscent of a typical [https://en.wikipedia.org/wiki/Serine_protease serine protease].<ref name="DesJarlais, R., & Tummino, P. J.">DesJarlais, R., & Tummino, P. J. (2016). Role of histone-modifying enzymes and their complexes in regulation of chromatin biology. Biochemistry, 55(11), 1584-1599. https://doi.org/10.1021/acs.biochem.5b01210 </ref> While Classes I, II, and IV do have some major distinctions such as size of the protein, in general, they share homology at the catalytic site. HDAC 8 is classified as a Class I HDAC alongside HDACs 1-3. In fact, within Class I HDACs, there are many invariant residues involved in the catalytic site (such as His-Asp dyads), Zn-binding, and ligand binding pocket (such as Asp101) (Figure 1). <ref name="Vannini, A., Volpari, C., Gallinari, P.">Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047 </ref> | ||
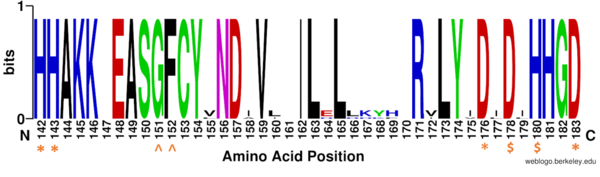
| - | [[Image:Conserved residues.PNG|600px||center||thumb|Figure 1: Weblogo representation comparing conservation of residues (143-182 in HDAC8) to homologous sequences in all class I HDACs. Nearly all active site residues (asterisk), zinc binding (dollar), and binding pocket residues (caret) are conserved across all class I HDACs. Other conserved residues not shown include active site residue Tyr306, zinc binding residue Asp267, and binding pocket residue Asp101. Nonconserved residues from 158 to | + | [[Image:Conserved residues.PNG|600px||center||thumb|Figure 1: Weblogo representation comparing conservation of residues (143-182 in HDAC8) to homologous sequences in all class I HDACs. Nearly all active site residues (asterisk), zinc binding (dollar), and binding pocket residues (caret) are conserved across all class I HDACs. Other conserved residues not shown include active site residue Tyr306, zinc binding residue Asp267, and binding pocket residue Asp101. Nonconserved residues from 158 to 170 are part of an α-helix that moves outward from the active side before looping back around to the active site.]] |
==HDAC8 Structure== | ==HDAC8 Structure== | ||
| Line 35: | Line 35: | ||
==Mechanism== | ==Mechanism== | ||
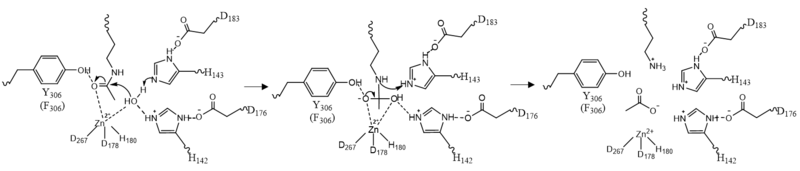
| - | Once the substrate is bound to the binding pocket through interactions with <scene name='81/811087/Ligand_interaction/4'>Asp101,Phe152 and Phe208</scene>, the water molecule attacks the carbonyl carbon of the ε-amino-lysine sidechain of N-terminal core of histone proteins (Figure 2). This water molecule is recruited and stabilized by <scene name='81/811085/Dyads/5'>two catalytic dyads</scene>. The first dyad consists of His143 and Asp183. Asp183 interacts with His143 to shift electron density so that His143 may act as a general base to remove a proton from water. The second catalytic dyad consists of His142 and Asp176 and stabilizes the now deprotonated water molecule. A Zn<sup>2+</sup> ion also makes the water more acidic making it a better nucleophile. The tetrahedral intermediate is stabilized by the Zn<sup>2+</sup> ion as well as Tyr306. The amine group of the substrate's lysine acts as a general base and deprotonates His143. This drives the tetrahedral intermediate to collapse and expel the acetyl group to produce an acetate ion and a deacetylated lysine residue. <ref name="Seto, E., & Yoshida, M."> Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology, 6(4), a018713. https://doi.org/10.1101/cshperspect.a018713 </ref> | + | Once the substrate is bound to the binding pocket through interactions with <scene name='81/811087/Ligand_interaction/4'>Asp101, Phe152 and Phe208</scene>, the water molecule attacks the carbonyl carbon of the ε-amino-lysine sidechain of N-terminal core of histone proteins (Figure 2). This water molecule is recruited and stabilized by <scene name='81/811085/Dyads/5'>two catalytic dyads</scene>. The first dyad consists of His143 and Asp183. Asp183 interacts with His143 to shift electron density so that His143 may act as a general base to remove a proton from water. The second catalytic dyad consists of His142 and Asp176 and stabilizes the now deprotonated water molecule. A Zn<sup>2+</sup> ion also makes the water more acidic making it a better nucleophile. The tetrahedral intermediate is stabilized by the Zn<sup>2+</sup> ion as well as Tyr306. The amine group of the substrate's lysine acts as a general base and deprotonates His143. This drives the tetrahedral intermediate to collapse and expel the acetyl group to produce an acetate ion and a deacetylated lysine residue. <ref name="Seto, E., & Yoshida, M."> Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology, 6(4), a018713. https://doi.org/10.1101/cshperspect.a018713 </ref> |
[[Image:Mech.PNG|800px||center||thumb|Figure 2: HDAC8 Mechanism: Tyr306 was mutated to Phe306 to determine the crystal structure in the pdb file 2v5w.]] | [[Image:Mech.PNG|800px||center||thumb|Figure 2: HDAC8 Mechanism: Tyr306 was mutated to Phe306 to determine the crystal structure in the pdb file 2v5w.]] | ||
| Line 44: | Line 44: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | ==Contributors== | ||
| + | Asif Hossain | ||
| + | Sean O'Brien | ||
| + | Josephine Thestrup | ||
Revision as of 17:48, 26 April 2019
Histone Deacetylase 8 (HDAC 8)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Vannini, A., Volpari, C., Gallinari, P., Jones, P., Mattu, M., Carfí, A., ... & Di Marco, S. (2007). Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8–substrate complex. EMBO reports, 8(9), 879-884. https://doi.org/10.1038/sj.embor.7401047
- ↑ DesJarlais, R., & Tummino, P. J. (2016). Role of histone-modifying enzymes and their complexes in regulation of chromatin biology. Biochemistry, 55(11), 1584-1599. https://doi.org/10.1021/acs.biochem.5b01210
- ↑ 3.0 3.1 3.2 3.3 3.4 Somoza J, Skene R. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure, 12(7), 1325-1334.2004. https://doi.org/10.1016/j.str.2004.04.012
- ↑ Whitehead, L., Dobler, M. R., Radetich, B., Zhu, Y., Atadja, P. W., Claiborne, T., ... & Shao, W. (2011). Human HDAC isoform selectivity achieved via exploitation of the acetate release channel with structurally unique small molecule inhibitors. Bioorganic & medicinal chemistry, 19(15), 4626-4634. https://doi.org/10.1016/j.bmc.2011.06.030
- ↑ 5.0 5.1 5.2 Vannini, A., Volpari, C., Filocamo, G., Casavola, E. C., Brunetti, M., Renzoni, D., ... & Steinkühler, C. (2004). Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proceedings of the National Academy of Sciences, 101(42), 15064-15069. https://dx.doi.org/10.1073%2Fpnas.0404603101
- ↑ Seto, E., & Yoshida, M. (2014). Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology, 6(4), a018713. https://doi.org/10.1101/cshperspect.a018713
- ↑ 7.0 7.1 Eckschlager T, Plch, J, Stiborova M, Hrabeta J.Histone deacetylase inhibitors as anticancer drugs. International journal of molecular sciences, 18(7), 1414. 2017. https://dx.doi.org/10.3390%2Fijms18071414
Contributors
Asif Hossain Sean O'Brien Josephine Thestrup