User:Caitlin Marie Gaich/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
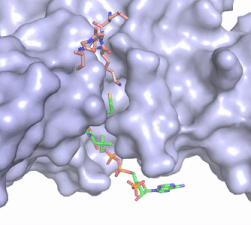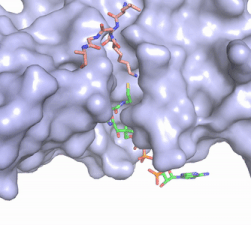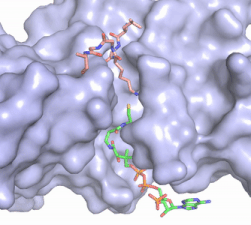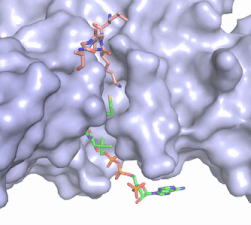
<StructureSection load='4PSW' size='350' frame='true' side='right' caption='HAT1/HAT2 Complex pdb: 4PSW' scene='81/811717/Overview/1'> | <StructureSection load='4PSW' size='350' frame='true' side='right' caption='HAT1/HAT2 Complex pdb: 4PSW' scene='81/811717/Overview/1'> | ||
=Histones= | =Histones= | ||
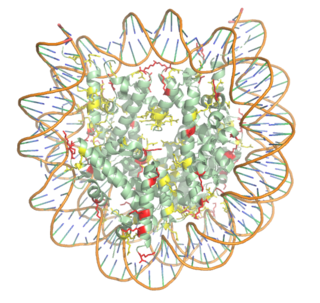
| - | [https://en.wikipedia.org/wiki/Histone Histones] are proteins found in the nucleus that are the key building blocks of [https://en.wikipedia.org/wiki/Chromatin chromatin] and are essential for proper DNA packaging and [https://en.wikipedia.org/wiki/Transcription_(biology) transcription]. In the first step of [https://www.hhmi.org/biointeractive/how-dna-packaged DNA packaging], two copies of the four core histone proteins ([https://en.wikipedia.org/wiki/Histone_H1 H1], [https://en.wikipedia.org/wiki/Histone_H2A H2A], [https://en.wikipedia.org/wiki/Histone_H3 H3], and [https://en.wikipedia.org/wiki/Histone_H4 H4]) form an [https://en.wikipedia.org/wiki/Histone_octamer octamer] in which DNA directly interacts with and wraps around, forming the [https://en.wikipedia.org/wiki/Nucleosome nucleosome]. 20-24% of residues making up the histone octamer are arginine and lysine, causing a net positive charge, especially at the outer surfaces of the histone core where negatively-charged DNA is bound <ref> Watson, J D, et al. Molecular Biology of the Gene (Seventh Edition). (2014) Boston, MA: Benjamin-Cummings Publishing Company. </ref> (Figure 1)<ref name="Watanabe"> PMID: 20100606 </ref> . After translation, the positively charged tails of the histone core that are often subject to modifications, such as acetylation or methylation. These modifications can regulate the processes of DNA repair, replication, transcription, and heterochromatin maintenance. | + | [https://en.wikipedia.org/wiki/Histone Histones] are proteins found in the nucleus that are the key building blocks of [https://en.wikipedia.org/wiki/Chromatin chromatin] and are essential for proper DNA packaging and [https://en.wikipedia.org/wiki/Transcription_(biology) transcription]. In the first step of [https://www.hhmi.org/biointeractive/how-dna-packaged DNA packaging], two copies of the four core histone proteins ([https://en.wikipedia.org/wiki/Histone_H1 H1], [https://en.wikipedia.org/wiki/Histone_H2A H2A], [https://en.wikipedia.org/wiki/Histone_H3 H3], and [https://en.wikipedia.org/wiki/Histone_H4 H4]) form an [https://en.wikipedia.org/wiki/Histone_octamer octamer] in which DNA directly interacts with and wraps around, forming the [https://en.wikipedia.org/wiki/Nucleosome nucleosome]. 20-24% of residues making up the histone octamer are arginine and lysine, causing a net positive charge, especially at the outer surfaces of the histone core where negatively-charged DNA is bound <ref> Watson, J D, et al. Molecular Biology of the Gene (Seventh Edition). (2014) Boston, MA: Benjamin-Cummings Publishing Company. </ref> (Figure 1) <ref name="Watanabe"> PMID: 20100606 </ref> . After translation, the positively charged tails of the histone core that are often subject to modifications, such as acetylation or methylation. These modifications can regulate the processes of DNA repair, replication, transcription, and heterochromatin maintenance. |
[[Image:Histone_NEWEST_w_DNA.png|320 px|right|thumb|Figure 1. Nucleosome consisting of the Histone core & DNA bound. Arginine residues shown in yellow. Lysine residues shown in red. PDB: 3kwq]] | [[Image:Histone_NEWEST_w_DNA.png|320 px|right|thumb|Figure 1. Nucleosome consisting of the Histone core & DNA bound. Arginine residues shown in yellow. Lysine residues shown in red. PDB: 3kwq]] | ||
=Histone Modification= | =Histone Modification= | ||
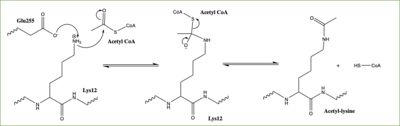
| - | Histones can be modified in a variety of ways, including: methylation, demethylation, acetylation, deacetylation and many others, all leading to either the condensation or relaxation of DNA and as a consequence turning on or off DNA transcription. Histone acetylation is histone modification that involves the transfer of an acetyl group from Acetyl Coenzyme A (acetyl-CoA) to an ε-amino group of a lysine residue on a histone. This reaction is done by various histone acetyltransferase (HAT) enzymes. The specific histone acetylation modification is an important [https://en.wikipedia.org/wiki/Epigenetics epigenetic] marker. It plays a role in RNA synthesis and there a known correlation between gene activity and histone acetylation. Any misregulations of the HAT enzyme can possibly lead to cancer, cardiovascular disease, and HIV <ref name="Ngo"> PMID: 30637990 </ref>. | + | Histones can be modified in a variety of ways, including: methylation, demethylation, acetylation, deacetylation and many others, all leading to either the condensation or relaxation of DNA and as a consequence turning on or off DNA transcription. Histone acetylation is histone modification that involves the transfer of an acetyl group from Acetyl Coenzyme A (acetyl-CoA) to an ε-amino group of a lysine residue on a histone. This reaction is done by various histone acetyltransferase (HAT) enzymes. The specific histone acetylation modification is an important [https://en.wikipedia.org/wiki/Epigenetics epigenetic] marker. It plays a role in RNA synthesis and there a known correlation between gene activity and histone acetylation. Any misregulations of the HAT enzyme can possibly lead to cancer, cardiovascular disease, and HIV <ref name= "Ngo"> PMID: 30637990 </ref>. |
=HAT1 Background = | =HAT1 Background = | ||
Revision as of 18:32, 26 April 2019
Histone Acetyltransferase HAT1/HAT2 Complex, Saccharomyces cerevisiae
| |||||||||||