We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Courtney Brown/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 33: | Line 33: | ||
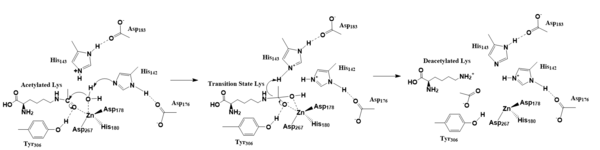
The zinc ion <scene name='81/811715/Zn_activating_water/4'>activates the water</scene> by withdrawing electron density from the water due to the positive charge, making the water more acidic, therefore a better nucleophile. The zinc ion also coordinates to the carbonyl oxygen of the acetyl group on the lysine, polarizing the carbonyl carbon, making it more electrophilic. There are two <scene name='81/811715/His_142_and_143/3'>His-Asp dyads</scene> present in the active site. His142 deprotonates the water, the first step of the deacetylation (Figure 2). His 142 (stabilized by Asp 176) is closer to the water molecule than His 143 (stabilized by Asp 183), which was also thought to deprotonate the water. However, a mutation done to His143 only reduced activity, not abolished it, showing it is important but not crucial. His143 is instead thought to orient the substrate<ref name="Vanninni" />. | The zinc ion <scene name='81/811715/Zn_activating_water/4'>activates the water</scene> by withdrawing electron density from the water due to the positive charge, making the water more acidic, therefore a better nucleophile. The zinc ion also coordinates to the carbonyl oxygen of the acetyl group on the lysine, polarizing the carbonyl carbon, making it more electrophilic. There are two <scene name='81/811715/His_142_and_143/3'>His-Asp dyads</scene> present in the active site. His142 deprotonates the water, the first step of the deacetylation (Figure 2). His 142 (stabilized by Asp 176) is closer to the water molecule than His 143 (stabilized by Asp 183), which was also thought to deprotonate the water. However, a mutation done to His143 only reduced activity, not abolished it, showing it is important but not crucial. His143 is instead thought to orient the substrate<ref name="Vanninni" />. | ||
| - | The attack by the deprotonated water forces the carbonyl carbon into a tetrahedral transition state. <scene name='81/811715/Tyr306/7'>Tyr306</scene><ref name="Vanninni">PMID:17721440</ref> acts as the oxyanion hole, stabilizing this transition state via its protruding -OH group hydrogen-bonding with the negatively charged oxygen. The transition state collapses, and His 143 simultaneously gets deprotonated by the amide of the leaving acetyl group in order to weaken the scissile bond. Breaking this scissile bond results in a neutral lysine and an | + | The attack by the deprotonated water forces the carbonyl carbon into a tetrahedral transition state. <scene name='81/811715/Tyr306/7'>Tyr306</scene><ref name="Vanninni">PMID:17721440</ref> acts as the oxyanion hole, stabilizing this transition state via its protruding -OH group hydrogen-bonding with the negatively charged oxygen. The transition state collapses, and His 143 simultaneously gets deprotonated by the amide of the leaving acetyl group in order to weaken the scissile bond. Breaking this scissile bond results in a neutral lysine and an acetic acid, among other products (Figure 2). The deacetylated lysine will be protinated by the hydrogen lost from this acetic acid, providing the hydrogen necessary for lysine to become basic, and forming an acetate ion<ref name="Vanninni" />. The residues that come in contact with the acetylated lysine, with the exception of Tyr306, are conserved (Figure 1), emphasizing how crucial these specific residues are in the function of an HDAC<ref name="Vanninni" />. |
==Inhibition== | ==Inhibition== | ||
Revision as of 20:06, 26 April 2019
The Human Histone Deacetylase, HDAC8
| |||||||||||
References
- ↑ 1.0 1.1 Histones | Learn Science at Scitable https://www.nature.com/scitable/definition/histone-histones-57
- ↑ What is chromatin, heterochromatin and euchromatin? MBInfo https://www.mechanobio.info/genome-regulation/what-is-chromatin-heterochromatin-and-euchromatin
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Vannini A, Volpari C, Gallinari P, Jones P, Mattu M, Carfi A, De Francesco R, Steinkuhler C, Di Marco S. Substrate binding to histone deacetylases as shown by the crystal structure of the HDAC8-substrate complex. EMBO Rep. 2007 Sep;8(9):879-84. Epub 2007 Aug 10. PMID:17721440
- ↑ Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014 Apr 1;6(4):a018713. doi:, 10.1101/cshperspect.a018713. PMID:24691964 doi:http://dx.doi.org/10.1101/cshperspect.a018713
- ↑ Chen K, Zhang X, Wu YD, Wiest O. Inhibition and mechanism of HDAC8 revisited. J Am Chem Soc. 2014 Aug 20;136(33):11636-43. doi: 10.1021/ja501548p. Epub 2014, Aug 7. PMID:25060069 doi:http://dx.doi.org/10.1021/ja501548p
- ↑ Tabackman AA, Frankson R, Marsan ES, Perry K, Cole KE. Structure of 'linkerless' hydroxamic acid inhibitor-HDAC8 complex confirms the formation of an isoform-specific subpocket. J Struct Biol. 2016 Sep;195(3):373-8. doi: 10.1016/j.jsb.2016.06.023. Epub 2016, Jun 29. PMID:27374062 doi:http://dx.doi.org/10.1016/j.jsb.2016.06.023
- ↑ 7.0 7.1 Marks PA. Histone deacetylase inhibitors: a chemical genetics approach to understanding cellular functions. Biochim Biophys Acta. 2010 Oct-Dec;1799(10-12):717-25. doi:, 10.1016/j.bbagrm.2010.05.008. Epub 2010 Jun 8. PMID:20594930 doi:http://dx.doi.org/10.1016/j.bbagrm.2010.05.008
- ↑ Vannini A, Volpari C, Filocamo G, Casavola EC, Brunetti M, Renzoni D, Chakravarty P, Paolini C, De Francesco R, Gallinari P, Steinkuhler C, Di Marco S. Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc Natl Acad Sci U S A. 2004 Oct 19;101(42):15064-9. Epub 2004 Oct 11. PMID:15477595
Student Contributors
- Courtney Brown
- Cassandra Marsh
- Carolyn Hurdle