We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Madeleine Wilson/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
==Inhibitors== | ==Inhibitors== | ||
| - | + | ||
| - | Sinefungin is a potent methyltransferase inhibitor. | + | SET7/9’s structure and function has been studied extensively because of its role in transcription <ref name="Takemoto">PMID:27088648</ref>. In the past few years it has been identified to methylate genes involved in multiple diseases; making it a potential candidate for drug inhibition. <ref name="Tamura">PMID:29723250</ref> Two compounds that have been found to inhibit SET7/9 in certain cells in vitro are Sinefungin and Cyproheptadine. Each inhibitor acts on the catalytic center of SET7/9, however their mechanisms of inhibition and possible medical relevancies differ greatly. |
| + | |||
| + | ===Sinefungin=== | ||
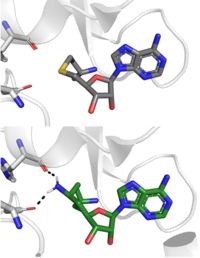
| + | Sinefungin is a potent methyltransferase inhibitor that is a natural nucleoside isolated from the [https://www.britannica.com/science/Streptomyces "Streptomyces"] species. <ref name="Tamura" /> Also referred to as adenosyl-ornithine, it is the delta (5’ adenosyl) derivative of [https://en.wikipedia.org/wiki/Ornithine ornithine] and a [https://en.wikipedia.org/wiki/Structural_analog structural analog] of S-adenosylmethionine. Sinefugin is unique because it inhibits where the cofactor binds rather than where the substrate binds like a typical competitive inhibitor. Sinefungin is more stable bound in the active site than SAH due to the ability to create two additional hydrogen bonds to its amine group that are not possible with SAH’s sulfur. | ||
| + | |||
| + | [[Image: SinSAH.jpg|200 px| right| thumb|SAH (grey) and Sinefungin (green) in the peptide binding pocket. The nitrogen group of sinefungin makes 2 double bonds to the main chain carbonyls of Arg265 and His293. Sinefungin was created using PDB: 1O9S and mutating the sulfur of SAH]] | ||
| + | |||
| + | Sinefungin has been used experimentally to inhibit the SET 7/9 protein on [https://www.sciencedirect.com/topics/medicine-and-dentistry/peritoneal-fibrosis peritoneal fibrosis] in mice and in human peritoneal mesothelial cells. <ref name="Tamura" /> SET 7/9 is involved in peritoneal fibrosis because it mono-methylates [https://epigenie.com/key-epigenetic-players/histone-proteins-and-modifications/histone-h3k4/ H3K4], which activates the transcription of fibrosis related genes. The administration of Sinefungin to mice in vitro resulted in decreased levels of methylated H3K4 (H3K4me1) protein, as well as suppressed peritoneal cell density and thickening. The decreased levels of H3K4me1 suggest that the methylation of H3K4 was inhibited by Sinefungin, as well as that inhibiting SET7/9 ameliorates peritoneal fibrosis. | ||
| + | |||
| + | ===Cyproheptadine=== | ||
| + | Another inhibitor of SET 7/9 is <scene name='81/811086/Cyproheptadine/3'>cyproheptadine</scene>, a clinically-approved anti-allergy drug that was originally developed as a serotonin and histimine. <ref name="Takemoto" /> The cyproheptadine-SET 7/9 complex was crystallized via X-ray diffraction at 2.005 Å with methylated cofactor SAM and with cydroheptadine. Unlike Sinefungin, it is a traditional competitor and competitive with the peptide substrates as it binds to the peptide-binding site. When cyproheptadine binds to the substrate site, the nitrogen of the [https://www.koeichem.com/en/product/index.php/item?cell003=Amines&cell004=Piperidine+derivatives&page=8&name=N-Methylpiperidine&id=116&label=1 methylpiperdine] ring of cyproheptadine forms a hydrogen bond with Thr286 as well as hydrophobic and interactions with the residues surrounding its binding site. The binding of cyproheptadine to the catalytic site causes conformational changes of residue Tyr337, an important residue for the formation of the lysine access channel. This movement subsequently causes a conformational change of the <scene name='81/811086/Betahairincypr/1'>beta hairpin</scene>. The <scene name='81/811086/Betahairpincyp/3'>residues 337-349</scene> conformational change ultimately generates a large hole adjacent to the lysine access channel, as well as the shift of the C-terminal helix. | ||
| + | With the revelation of its inhibitory effects on SET7/9, cyproheptadine was used in vitro to treat breast cancer cells ([https://en.wikipedia.org/wiki/MCF-7 MCF-7] cells). SET 7/9's non-histone activities include the methylation of the estrogen receptor α (ERα), a nuclear receptor and a transcription factor responsible for estrogen-responsive gene regulation. The expression and transcriptional activity of ERα is involved in the carcinogenesis of 70% of breast cancers, making it a major target for hormone therapy. Researchers found that treating the MCF7 cells with cyproheptadine decreased ERα's expression and transcriptional activity which therefore inhibited the estrogen-dependent cell growth. These findings suggest that cyproheptadine could possibly be repurposed to breast cancer therapy in the future. | ||
| + | |||
</StructureSection> | </StructureSection> | ||
Revision as of 23:30, 26 April 2019
Histone Lysine Methyltransferase: Gene Activator
| |||||||||||
References
- ↑ DesJarlais R, Tummino PJ. Role of Histone-Modifying Enzymes and Their Complexes in Regulation of Chromatin Biology. Biochemistry. 2016 Mar 22;55(11):1584-99. doi: 10.1021/acs.biochem.5b01210. Epub , 2016 Jan 26. PMID:26745824 doi:http://dx.doi.org/10.1021/acs.biochem.5b01210
- ↑ 2.0 2.1 doi: https://dx.doi.org/10.1016/j.apsb.2013.04.007
- ↑ 3.0 3.1 Dong X, Weng Z. The correlation between histone modifications and gene expression. Epigenomics. 2013 Apr;5(2):113-6. doi: 10.2217/epi.13.13. PMID:23566087 doi:http://dx.doi.org/10.2217/epi.13.13
- ↑ 4.0 4.1 Del Rizzo PA, Trievel RC. Substrate and product specificities of SET domain methyltransferases. Epigenetics. 2011 Sep 1;6(9):1059-67. doi: 10.4161/epi.6.9.16069. Epub 2011 Sep, 1. PMID:21847010 doi:http://dx.doi.org/10.4161/epi.6.9.16069
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003 Feb 6;421(6923):652-6. Epub 2003 Jan 22. PMID:12540855 doi:10.1038/nature01378
- ↑ 6.0 6.1 Kwon T, Chang JH, Kwak E, Lee CW, Joachimiak A, Kim YC, Lee J, Cho Y. Mechanism of histone lysine methyl transfer revealed by the structure of SET7/9-AdoMet. EMBO J. 2003 Jan 15;22(2):292-303. PMID:12514135 doi:http://dx.doi.org/10.1093/emboj/cdg025
- ↑ 7.0 7.1 Takemoto Y, Ito A, Niwa H, Okamura M, Fujiwara T, Hirano T, Handa N, Umehara T, Sonoda T, Ogawa K, Tariq M, Nishino N, Dan S, Kagechika H, Yamori T, Yokoyama S, Yoshida M. Identification of Cyproheptadine as an Inhibitor of SET Domain Containing Lysine Methyltransferase 7/9 (Set7/9) That Regulates Estrogen-Dependent Transcription. J Med Chem. 2016 Apr 28;59(8):3650-60. doi: 10.1021/acs.jmedchem.5b01732. Epub, 2016 Apr 18. PMID:27088648 doi:http://dx.doi.org/10.1021/acs.jmedchem.5b01732
- ↑ 8.0 8.1 8.2 Tamura R, Doi S, Nakashima A, Sasaki K, Maeda K, Ueno T, Masaki T. Inhibition of the H3K4 methyltransferase SET7/9 ameliorates peritoneal fibrosis. PLoS One. 2018 May 3;13(5):e0196844. doi: 10.1371/journal.pone.0196844., eCollection 2018. PMID:29723250 doi:http://dx.doi.org/10.1371/journal.pone.0196844
Student Contributors
Lauryn Padgett, Alexandra Pentala, Madeleine Wilson