User:Tereza Čalounová/Sandbox 1
From Proteopedia
m |
m |
||
| Line 45: | Line 45: | ||
==== Remodeling ==== | ==== Remodeling ==== | ||
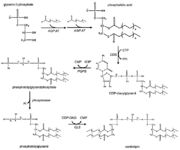
| + | [[Image:RemodelingCardiolipin.png|thumb|Cardiolipin synthesis and remodeling.]] | ||
The remodeling is based on unsaturation of the acyl chains of the cardiolipin. This process has two steps. Firstly, the fatty acyl group is removed by a specific cardiolipin deacylase forming monolysocardiolipin. Then tafazzin recyclates monolysocardiolipin and makes the final product, unsaturated cardiolipin. <ref name="cit15" /> | The remodeling is based on unsaturation of the acyl chains of the cardiolipin. This process has two steps. Firstly, the fatty acyl group is removed by a specific cardiolipin deacylase forming monolysocardiolipin. Then tafazzin recyclates monolysocardiolipin and makes the final product, unsaturated cardiolipin. <ref name="cit15" /> | ||
| Line 80: | Line 81: | ||
== 3D Structure: Homology Model == | == 3D Structure: Homology Model == | ||
| - | No empirical 3D structure for human tafazzin protein (UniProt Q16635) is available in April, 2019. In view of this, homology model was constructed using Swiss-Model server. The closest related protein to tafazzin with a known 3D structure in a public database is in April 2019 plant glycerol 3-phosphate acyltransferase (G3PAT) which has sequence identity of 18,10 %, sequence similarity is 0,29. Tafazzin belongs to a protein superfamily - phospholipid acyltransferases (PF01553). | + | No empirical 3D structure for human tafazzin protein (UniProt Q16635) is available in April, 2019. In view of this, homology model was constructed using Swiss-Model server. <ref name="cit17">https://swissmodel.expasy.org/repository/uniprot/Q16635</ref> The closest related protein to tafazzin with a known 3D structure in a public database is in April 2019 plant glycerol 3-phosphate acyltransferase (G3PAT) which has sequence identity of 18,10 %, sequence similarity is 0,29. Tafazzin belongs to a protein superfamily - phospholipid acyltransferases (PF01553). |
| - | Domains found on sequence of human tafazzin contain transmembrane domain (15-34) and acyltransferase domain (41-215). <ref name="cit11">http://pfam.xfam.org/protein/Q16635</ref> In the acyltransferase domain there is a HX<sub>4</sub>D motif - histidine and aspartic acid residues separated by four less conserved residues. Sequence analysis of membrane-bound glycerolipid acyltransferases revealed that proteins from the bacterial, plant, and animal kingdoms share this highly conserved domain containing HX<sub>4</sub>D motif. | + | Domains found on sequence of human tafazzin contain transmembrane domain (15-34) and acyltransferase domain (41-215). <ref name="cit11">http://pfam.xfam.org/protein/Q16635</ref> |
| + | [[Image:ConSurfTafazzin.png|thumb|Output from ConSurf analysis of tafazzin showing HX4D motif conservation.]] | ||
| + | [[Image:HMMLogoAcyltransferase.png|thumb|HMM logo of acyltransferase family (PF01553). <ref name="cit18">https://pfam.xfam.org/family/PF01553#tabview=tab4</ref>]] | ||
| + | |||
| + | In the acyltransferase domain there is a HX<sub>4</sub>D motif - histidine and aspartic acid residues separated by four less conserved residues. Sequence analysis of membrane-bound glycerolipid acyltransferases revealed that proteins from the bacterial, plant, and animal kingdoms share this highly conserved domain containing HX<sub>4</sub>D motif. | ||
. Aspartic His 69 – Asp 74 PŘIDAT SCÉNU | . Aspartic His 69 – Asp 74 PŘIDAT SCÉNU | ||
| + | Hijikatu, Yura, Ohara and Go in their work from 2015 predicted the intrinsically unstructured regions present in human tafazzin using 15 available prediction servers, eleven of them consistently predicted that the region encoded by exon 5 is intrinsically unstructured, and no other regions of the tafazzin sequence were consistently predicted as intrinsically unstructured. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 19:40, 27 April 2019
This is our page where we will share informations about protein Tafazzin as a part of a school project with my classmates. This page is under a construction so please be aware of it. Zde přidám úvod
Tafazzin
| Theoretical Model: The protein structure described on this page was determined theoretically, and hence should be interpreted with caution. |
Tafazzin is a protein located in mitochondrial inner membranes. It is involved in altering cardiolipin. Cardiolipin is key in maintaining mitochondrial shape, energy production, and protein transport within cells. The full-length tafazzin protein contains 292 amino acids and has a molecular weight of 33459 daltons. Mutations in gene associated with this protein can cause Barth Syndrome. Barth syndrome (BTHS),is a genetic disorder diagnosed almost exclusively in males. BTHS is rare, it is estimated to affect 1 in 300,000 to 400,000 individuals worldwide. Males with BTHS have weak heart and skeletal muscles which can lead to heart failure. Another of the symptoms is neutropenia which can lead to infections. [1]
| |||||||||||
References
- ↑ https://ghr.nlm.nih.gov/condition/barth-syndrome#
- ↑ 2.0 2.1 https://www.uniprot.org/uniprot/Q16635?fbclid=IwAR3v10lUTRZfb0NFOYKC4wjaherdU9PIVJ8T63jkC9RfNu_5OQ2IpoDR0iY
- ↑ 3.0 3.1 3.2 3.3 http://www.lipidhome.co.uk/lipids/complex/dpg/index.htm
- ↑ 4.0 4.1 4.2 4.3 https://link.springer.com/article/10.1007%2Fs00018-008-8030-5
- ↑ https://commons.wikimedia.org/wiki/File:Cardiolipin_structure.svg?uselang=cs
- ↑ https://commons.wikimedia.org/wiki/File:Eukaryotic_pathway.jpg
- ↑ 7.0 7.1 7.2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4342993/
- ↑ https://ghr.nlm.nih.gov/gene/TAZ#location
- ↑ 9.0 9.1 https://www.uniprot.org/uniprot/Q16635
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4412953/
- ↑ 11.0 11.1 https://ghr.nlm.nih.gov/condition/barth-syndrome#
- ↑ 12.0 12.1 https://www.sciencedirect.com/science/article/pii/S092544391830334X?via%3Dihub
- ↑ 13.0 13.1 13.2 https://obgyn.onlinelibrary.wiley.com/doi/full/10.1002/pd.2599
- ↑ https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/tafazzin
- ↑ 15.0 15.1 15.2 https://www.ncbi.nlm.nih.gov/books/NBK247162/
- ↑ https://swissmodel.expasy.org/repository/uniprot/Q16635
- ↑ http://pfam.xfam.org/protein/Q16635
- ↑ https://pfam.xfam.org/family/PF01553#tabview=tab4