User:Tereza Čalounová/Sandbox 1
From Proteopedia
m |
|||
| Line 64: | Line 64: | ||
=== Symptoms === | === Symptoms === | ||
| - | *dilated cardiomyopathy (CMD) | + | *[https://en.wikipedia.org/wiki/Dilated_cardiomyopathy dilated cardiomyopathy (CMD)] |
| - | *endocardial fibroelastosis (EFE) | + | *[https://en.wikipedia.org/wiki/Endocardial_fibroelastosis endocardial fibroelastosis (EFE)] |
*a predominantly proximal skeletal myopathy | *a predominantly proximal skeletal myopathy | ||
*growth retardation | *growth retardation | ||
*neutropenia | *neutropenia | ||
| - | *organic aciduria | + | *[https://en.wikipedia.org/wiki/Organic_acidemia organic aciduria] |
*excess of 3-methylglutaconic acid <ref name="cit5" /> | *excess of 3-methylglutaconic acid <ref name="cit5" /> | ||
*characteristic facial features (tall and broad forehead, round face, full cheeks, prominent pointed chin, large ears, and deep-set eyes) <ref name="cit10" /> | *characteristic facial features (tall and broad forehead, round face, full cheeks, prominent pointed chin, large ears, and deep-set eyes) <ref name="cit10" /> | ||
Revision as of 22:16, 27 April 2019
This is our page where we will share informations about protein Tafazzin as a part of a school project with my classmates. This page is under a construction so please be aware of it. Zde přidám úvod
Tafazzin
| Theoretical Model: The protein structure described on this page was determined theoretically, and hence should be interpreted with caution. |
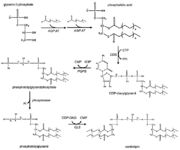
Tafazzin is a protein located in mitochondrial inner membranes. It is involved in altering cardiolipin. Cardiolipin is key in maintaining mitochondrial shape, energy production, and protein transport within cells. The full-length tafazzin protein contains 292 amino acids and has a molecular weight of 33459 daltons. Mutations in gene associated with this protein can cause Barth Syndrome. Barth syndrome (BTHS) is a genetic disorder diagnosed almost exclusively in males. BTHS is rare, it is estimated to affect 1 in 300,000 to 400,000 individuals worldwide. Males with BTHS have weak heart and skeletal muscles which can lead to heart failure. Another of the symptoms is neutropenia which can lead to infections. [1]
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Barth syndrome. In: Ghr.nlm.nih.gov [online]. Rockville Pike: U.S. National Library of Medicine, 2019 [cit. 2019-04-27]. Available at: https://ghr.nlm.nih.gov/condition/barth-syndrome#
- ↑ 2.0 2.1 2.2 2.3 Q16635 (TAZ_HUMAN). In: Https://www.uniprot.org/ [online]. Cambridge, Geneva, Washington: UniProt, 2019 [cit. 2019-04-27]. Available at: https://www.uniprot.org/uniprot/Q16635?fbclid=IwAR3v10lUTRZfb0NFOYKC4wjaherdU9PIVJ8T63jkC9RfNu_5OQ2IpoDR0iY
- ↑ 3.0 3.1 3.2 3.3 CHRISTIE, William. Cardiolipin (Diphosphatidylglycerol). In: Www.lipidhome.co.uk [online]. Hutton: The LipidWeb, 2019 [cit. 2019-04-27]. Available at: http://www.lipidhome.co.uk/lipids/complex/dpg/index.htm
- ↑ 4.0 4.1 4.2 4.3 Houtkooper, R. H., & Vaz, F. M. (2008). Cardiolipin, the heart of mitochondrial metabolism. Cellular and Molecular Life Sciences, 65(16), 2493–2506. doi:10.1007/s00018-008-8030-5
- ↑ Cardiolipin structure. In: Commons.wikimedia.org [online]. San Francisco: Wikimedia, 2019 [cit. 2019-04-27]. Available at: https://commons.wikimedia.org/wiki/File:Cardiolipin_structure.svg?uselang=cs
- ↑ Rochellehx. File:Eukaryotic pathway.jpg. In: Wikimedia Commons [online]. 23. 4. 2009 [cit. 2019-04-27]. Available at: https://commons.wikimedia.org/wiki/File:Eukaryotic_pathway.jpg
- ↑ 7.0 7.1 7.2 Raja V, Greenberg ML. The functions of cardiolipin in cellular metabolism-potential modifiers of the Barth syndrome phenotype. Chem Phys Lipids. 2014 Apr;179:49-56. doi: 10.1016/j.chemphyslip.2013.12.009., Epub 2014 Jan 17. PMID:24445246 doi:http://dx.doi.org/10.1016/j.chemphyslip.2013.12.009
- ↑ TAZ gene. In: Www.ncbi.nlm.nih.gov [online]. Rockville Pike: U.S. National Library of Medicine, 2014 [cit. 2019-04-27]. Available at: https://ghr.nlm.nih.gov/gene/TAZ#
- ↑ 9.0 9.1 Hijikata A, Yura K, Ohara O, Go M. Structural and functional analyses of Barth syndrome-causing mutations and alternative splicing in the tafazzin acyltransferase domain. Meta Gene. 2015 Apr 22;4:92-106. doi: 10.1016/j.mgene.2015.04.001. eCollection, 2015 Jun. PMID:25941633 doi:http://dx.doi.org/10.1016/j.mgene.2015.04.001
- ↑ 10.0 10.1 Barth syndrome cells display widespread remodeling of mitochondrial complexes without affecting metabolic flux distribution. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease [online]. 2018, 1864(11), 3650-3658 [cit. 2019-04-27]. Available at: https://www.sciencedirect.com/science/article/pii/S092544391830334X?via%3Dihub
- ↑ 11.0 11.1 Barth syndrome: an X‐linked cause of fetal cardiomyopathy and stillbirth. Prenatal Diagnosis [online]. 2010, 30(10), 970-976 [cit. 2019-04-27]. Available at: https://obgyn.onlinelibrary.wiley.com/doi/full/10.1002/pd.2599
- ↑ Tafazzin. In: Www.sciencedirect.com [online]. Amsterdam: Elsevier, 2014 [cit. 2019-04-27]. Available at: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/tafazzin
- ↑ 13.0 13.1 13.2 Ferreira C, Thompson R, Vernon H. Barth Syndrome PMID:25299040
- ↑ 14.0 14.1 Q16635 (TAZ_HUMAN) Homo sapiens (Human). In: Swissmodel.expasy.org/ [online]. Lausanne, Basel: swissmodel, 2019 [cit. 2019-04-27]. Available at: https://swissmodel.expasy.org/repository/uniprot/Q16635
- ↑ Family: Acyltransferase (PF01553). In: Pfam.xfam.org [online]. Heidelberg: EMBL [cit. 2019-04-27]. Available at: https://pfam.xfam.org/family/PF01553#tabview=tab0
- ↑ Protein: TAZ_HUMAN (Q16635). In: Pfam.xfam.org [online]. Heidelberg: EMBL [cit. 2019-04-27]. Available at: http://pfam.xfam.org/protein/Q16635
- ↑ Family: Acyltransferase (PF01553). In: Pfam.xfam.org [online]. Heidelberg: EMBL [cit. 2019-04-27]. Available at: https://pfam.xfam.org/family/PF01553#tabview=tab4
- ↑ Heath RJ, Rock CO. A conserved histidine is essential for glycerolipid acyltransferase catalysis. J Bacteriol. 1998 Mar;180(6):1425-30. PMID:9515909