User:Tereza Čalounová/Sandbox 1
From Proteopedia
m |
m |
||
| Line 44: | Line 44: | ||
==== Remodeling ==== | ==== Remodeling ==== | ||
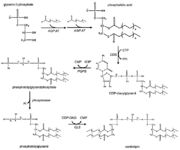
[[Image:RemodelingCardiolipin.png|thumb|Cardiolipin synthesis and remodeling.]] | [[Image:RemodelingCardiolipin.png|thumb|Cardiolipin synthesis and remodeling.]] | ||
| - | The remodeling is based on unsaturation of the acyl chains of the cardiolipin. This process has two steps. Firstly, the fatty acyl group is removed by a specific cardiolipin deacylase forming monolysocardiolipin. Then tafazzin | + | The remodeling is based on unsaturation of the acyl chains of the cardiolipin. This process has two steps. Firstly, the fatty acyl group is removed by a specific cardiolipin deacylase forming monolysocardiolipin. Then tafazzin recycles monolysocardiolipin and makes the final product, unsaturated cardiolipin. <ref name="cit15" /> |
==== Function ==== | ==== Function ==== | ||
Revision as of 11:06, 28 April 2019
This page is a project made with my classmates Kateřina Krausová and Dominik Merta for Structural Biology of the Cell Class in summer 2019 at the Charles University.
Tafazzin
| Theoretical Model: The protein structure described on this page was determined theoretically, and hence should be interpreted with caution. |
Tafazzin is a protein located in mitochondrial inner membranes. It is involved in altering cardiolipin. Cardiolipin is key in maintaining mitochondrial shape, energy production, and protein transport within cells. The full-length tafazzin protein contains 292 amino acids and has a molecular weight of 33459 daltons. Mutations in gene associated with this protein can cause Barth Syndrome. Barth syndrome (BTHS) is a genetic disorder diagnosed almost exclusively in males. BTHS is rare, it is estimated to affect 1 in 300,000 to 400,000 individuals worldwide. Males with BTHS have weak heart and skeletal muscles which can lead to heart failure. Another of the symptoms is neutropenia which can lead to infections. [1]
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Barth syndrome. In: Ghr.nlm.nih.gov [online]. Rockville Pike: U.S. National Library of Medicine, 2019 [cit. 2019-04-27]. Available at: https://ghr.nlm.nih.gov/condition/barth-syndrome#
- ↑ 2.0 2.1 2.2 2.3 Q16635 (TAZ_HUMAN). In: Https://www.uniprot.org/ [online]. Cambridge, Geneva, Washington: UniProt, 2019 [cit. 2019-04-27]. Available at: https://www.uniprot.org/uniprot/Q16635?fbclid=IwAR3v10lUTRZfb0NFOYKC4wjaherdU9PIVJ8T63jkC9RfNu_5OQ2IpoDR0iY
- ↑ 3.0 3.1 3.2 3.3 CHRISTIE, William. Cardiolipin (Diphosphatidylglycerol). In: Www.lipidhome.co.uk [online]. Hutton: The LipidWeb, 2019 [cit. 2019-04-27]. Available at: http://www.lipidhome.co.uk/lipids/complex/dpg/index.htm
- ↑ 4.0 4.1 4.2 4.3 Houtkooper, R. H., & Vaz, F. M. (2008). Cardiolipin, the heart of mitochondrial metabolism. Cellular and Molecular Life Sciences, 65(16), 2493–2506. doi:10.1007/s00018-008-8030-5
- ↑ Cardiolipin structure. In: Commons.wikimedia.org [online]. San Francisco: Wikimedia, 2019 [cit. 2019-04-27]. Available at: https://commons.wikimedia.org/wiki/File:Cardiolipin_structure.svg?uselang=cs
- ↑ Rochellehx. File:Eukaryotic pathway.jpg. In: Wikimedia Commons [online]. 23. 4. 2009 [cit. 2019-04-27]. Available at: https://commons.wikimedia.org/wiki/File:Eukaryotic_pathway.jpg
- ↑ 7.0 7.1 7.2 Raja V, Greenberg ML. The functions of cardiolipin in cellular metabolism-potential modifiers of the Barth syndrome phenotype. Chem Phys Lipids. 2014 Apr;179:49-56. doi: 10.1016/j.chemphyslip.2013.12.009., Epub 2014 Jan 17. PMID:24445246 doi:http://dx.doi.org/10.1016/j.chemphyslip.2013.12.009
- ↑ TAZ gene. In: Www.ncbi.nlm.nih.gov [online]. Rockville Pike: U.S. National Library of Medicine, 2014 [cit. 2019-04-27]. Available at: https://ghr.nlm.nih.gov/gene/TAZ#
- ↑ 9.0 9.1 Hijikata A, Yura K, Ohara O, Go M. Structural and functional analyses of Barth syndrome-causing mutations and alternative splicing in the tafazzin acyltransferase domain. Meta Gene. 2015 Apr 22;4:92-106. doi: 10.1016/j.mgene.2015.04.001. eCollection, 2015 Jun. PMID:25941633 doi:http://dx.doi.org/10.1016/j.mgene.2015.04.001
- ↑ 10.0 10.1 Barth syndrome cells display widespread remodeling of mitochondrial complexes without affecting metabolic flux distribution. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease [online]. 2018, 1864(11), 3650-3658 [cit. 2019-04-27]. Available at: https://www.sciencedirect.com/science/article/pii/S092544391830334X?via%3Dihub
- ↑ 11.0 11.1 Barth syndrome: an X‐linked cause of fetal cardiomyopathy and stillbirth. Prenatal Diagnosis [online]. 2010, 30(10), 970-976 [cit. 2019-04-27]. Available at: https://obgyn.onlinelibrary.wiley.com/doi/full/10.1002/pd.2599
- ↑ Tafazzin. In: Www.sciencedirect.com [online]. Amsterdam: Elsevier, 2014 [cit. 2019-04-27]. Available at: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/tafazzin
- ↑ 13.0 13.1 13.2 Ferreira C, Thompson R, Vernon H. Barth Syndrome PMID:25299040
- ↑ 14.0 14.1 Q16635 (TAZ_HUMAN) Homo sapiens (Human). In: Swissmodel.expasy.org/ [online]. Lausanne, Basel: swissmodel, 2019 [cit. 2019-04-27]. Available at: https://swissmodel.expasy.org/repository/uniprot/Q16635
- ↑ Family: Acyltransferase (PF01553). In: Pfam.xfam.org [online]. Heidelberg: EMBL [cit. 2019-04-27]. Available at: https://pfam.xfam.org/family/PF01553#tabview=tab0
- ↑ Protein: TAZ_HUMAN (Q16635). In: Pfam.xfam.org [online]. Heidelberg: EMBL [cit. 2019-04-27]. Available at: http://pfam.xfam.org/protein/Q16635
- ↑ Family: Acyltransferase (PF01553). In: Pfam.xfam.org [online]. Heidelberg: EMBL [cit. 2019-04-27]. Available at: https://pfam.xfam.org/family/PF01553#tabview=tab4
- ↑ Heath RJ, Rock CO. A conserved histidine is essential for glycerolipid acyltransferase catalysis. J Bacteriol. 1998 Mar;180(6):1425-30. PMID:9515909