Blue Luminescent Antibody Derived from House Mouse
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== Ligand Interactions == | == Ligand Interactions == | ||
| - | Ligands of this protein include glycerol and 4-(4-Styryl-Phenyl Carbamoyl)-Butyric Acid. In the figure, the three smaller molecules are the sites glycerol interaction and the larger molecules the site of 4-(4-Styryl-Phenyl Carbamoyl)-Butyric Acid. Glycerol is represented by GOL and 4-(4-Styryl-Phenyl Carbamoyl)-Butyric Acid is represented by SPB. GOL has significantly more conformations than SPB that can be bound by this protein, which is due to this protein having 3 separate binding sites for it. SPB only has two different conformations, and both are bound in the same binding pocket, although they are attached to two separate chains in the binding pocket. The four binding sites are shown in the linked figures, with example ligands in them. Note that one of the GOL binding sites is extremely close the the SPB binding site. The SPB binding site is on the deep interior of the protein, whereas all of the GOL binding sites are closer to the surface of the protein. GOL is bound by chains A, H, and L. SPB is bound by chains B and L. Specific ligand interactions are represented by SPB 301 L, GOL 402 L, GOL 406 L, GOL 405 H, GOL 401 A, GOL 403 A, GOL 404 A, and SPB 302 A. The first set of three letter is the ligand that is bound, the number represents the specific conformation of the ligand, and the last letter represents the chain being interacted with. | + | Ligands of this protein include glycerol and 4-(4-Styryl-Phenyl Carbamoyl)-Butyric Acid<ref name="pdb" />. In the figure, the three smaller molecules are the sites glycerol interaction and the larger molecules the site of 4-(4-Styryl-Phenyl Carbamoyl)-Butyric Acid. Glycerol is represented by GOL and 4-(4-Styryl-Phenyl Carbamoyl)-Butyric Acid is represented by SPB. GOL has significantly more conformations than SPB that can be bound by this protein, which is due to this protein having 3 separate binding sites for it. SPB only has two different conformations, and both are bound in the same binding pocket, although they are attached to two separate chains in the binding pocket. The four binding sites are shown in the linked figures, with example ligands in them. Note that one of the GOL binding sites is extremely close the the SPB binding site. The SPB binding site is on the deep interior of the protein, whereas all of the GOL binding sites are closer to the surface of the protein. GOL is bound by chains A, H, and L. SPB is bound by chains B and L. Specific ligand interactions are represented by SPB 301 L, GOL 402 L, GOL 406 L, GOL 405 H, GOL 401 A, GOL 403 A, GOL 404 A, and SPB 302 A. The first set of three letter is the ligand that is bound, the number represents the specific conformation of the ligand, and the last letter represents the chain being interacted with. |
| Line 22: | Line 22: | ||
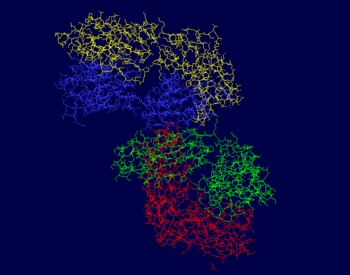
The heavy chains of this protein include the B and H chains. It is a total of 426 residues long. Both chains are 213 residues long and are L polypeptides. Chain B is 5% helical and 51% beta sheet, with 44% of its residues not having a secondary structure. Chain H is 5% helical and 50% beta sheet, with 45% of its residues not having a secondary structure. Chain B contains the binding site for SPB B 302, and Chain H has binding sites for both GOL H 405 and SPB L 301. | The heavy chains of this protein include the B and H chains. It is a total of 426 residues long. Both chains are 213 residues long and are L polypeptides. Chain B is 5% helical and 51% beta sheet, with 44% of its residues not having a secondary structure. Chain H is 5% helical and 50% beta sheet, with 45% of its residues not having a secondary structure. Chain B contains the binding site for SPB B 302, and Chain H has binding sites for both GOL H 405 and SPB L 301. | ||
| - | The photos to the right show that the majority of this protein is composed of beta sheets with the occasional alpha helix, however a good portion of this protein is not a part of a secondary structure. In the first figure, the A chain is pictured in yellow, the L chain in red, the B chain in blue, and the H chain in green. In the second figure, alpha helices are pictured in red while beta sheets are shown in yellow. The portions of 3CFB that do not form a secondary structure are shown by white strands. | + | The photos to the right show that the majority of this protein is composed of beta sheets with the occasional alpha helix, however a good portion of this protein is not a part of a secondary structure. In the first figure, the A chain is pictured in yellow, the L chain in red, the B chain in blue, and the H chain in green. In the second figure, alpha helices are pictured in red while beta sheets are shown in yellow. The portions of 3CFB that do not form a secondary structure are shown by white strands<ref name="pdb">. |
== Luminescent Quality == | == Luminescent Quality == | ||
Revision as of 15:10, 1 May 2019
| |||||||||||