Blue Luminescent Antibody Derived from House Mouse
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
<scene name='81/814060/Active_site_mouse/1'>Ligand Interactions of 3CFB</scene> | <scene name='81/814060/Active_site_mouse/1'>Ligand Interactions of 3CFB</scene> | ||
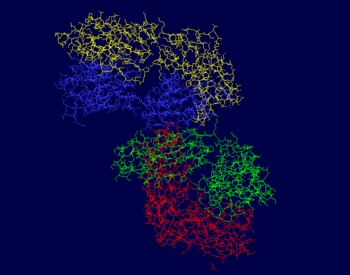
| - | The light chains of this protein include the A and L chains. The A chain is made up of 219 residues in total | + | The light chains of this protein include the A and L chains. The A chain is made up of 219 residues in total and is an L polypeptide. The A chain has 3 helices composed of 11 residues and 25 strands of beta sheets with 113 residues. This strand is 5% helical and 51% beta sheet, which means that 44% of this chain is not a part of a secondary structure. The L chain is also made up of 219 residues total and is a L polypeptide. The L chain has 3 helices with 12 residues total and 25 strands of beta sheets made up of 105 residues. This chain is 5% helical and 47% beta sheet, meaning that 48% of this chain is not part of a secondary structure. Chain A has binding sites for GOL A 403, GOL A 404, SPB B 302, and GOL A 401. Chain L has the binding sites for SPB L 301, GOL L 402, and GOL L 406. |
The heavy chains of this protein include the B and H chains. It is a total of 426 residues long. Both chains are 213 residues long and are L polypeptides. Chain B is 5% helical and 51% beta sheet, with 44% of its residues not having a secondary structure. Chain H is 5% helical and 50% beta sheet, with 45% of its residues not having a secondary structure. Chain B contains the binding site for SPB B 302, and Chain H has binding sites for both GOL H 405 and SPB L 301. | The heavy chains of this protein include the B and H chains. It is a total of 426 residues long. Both chains are 213 residues long and are L polypeptides. Chain B is 5% helical and 51% beta sheet, with 44% of its residues not having a secondary structure. Chain H is 5% helical and 50% beta sheet, with 45% of its residues not having a secondary structure. Chain B contains the binding site for SPB B 302, and Chain H has binding sites for both GOL H 405 and SPB L 301. | ||
| - | The photos to the right show that the majority of this protein is composed of beta sheets with the occasional alpha helix | + | The photos to the right show that the majority of this protein is composed of beta sheets with the occasional alpha helix; however, a good portion of this protein is not a part of a secondary structure. In the first figure, the A chain is pictured in yellow, the L chain in red, the B chain in blue, and the H chain in green. In the second figure, alpha helices are pictured in red, while beta sheets are shown in yellow. The portions of 3CFB that do not form a secondary structure are shown as white strands.<ref name="PDB" /> |
== Luminescent Quality == | == Luminescent Quality == | ||
| - | While this protein was originally known to be fluorescent, some important differences led | + | While this protein was originally known to be fluorescent, some important differences led researchers to believe that its actual light emitting mechanism was not that of fluorescence. For example, this protein gives off more light at higher temperatures, whereas a typical fluorescent dye gives off less light as the temperature increases. Most fluorescent molecules are non-organic, whereas this molecule is a protein and emits a light that is significantly brighter than non-organic fluorescent dyes. Although this protein was previously referred to as a fluorescent protein because of its light generation and its close relation to true fluorescent proteins, it is more properly referred to as luminescent or light emitting. Instead of the glow being caused by an excited electron’s decay into a lower energy state (as it would be in typical fluorescence), it is caused by a charge transfer reaction between a tryptophan residue and the trans-stilbene complex that undergoes deeply inverted electron hole recombination. This protein's rigid matrix allows this recombination to occur and allows this excited state to endure for a longer time period than is standard for light emitting proteins. Protein luminescence through deeply inverted electron hole recombination is very rare, and 3CFB is a very unique example of this. This electron transfer is the reason the antibody is referred to as luminescent instead of fluorescent, and this reaction can also be elucidated upon UV (ultraviolet) excitation. The luminescence of this protein is increased by two orders of magnitude as compared to other antibody-stilbene complexes. A related protein, PDB 3CFE, does emit a purple fluorescence. The blue color of 3CFB is due to a 30 nm shift in the light spectrum from that of 3CFE. The shift is mostly due to an altered tryptophan residue, which allows this antibody to emit such a bright light. This residue is also responsible for the duration of the blue light, as it gives the protein a luminescent quality instead of a fluorescent one. The protein 3CFE is in fact fluorescent, as it does not have a Trp residue interacting with the stilbene it is attached to. 3CFB also has a stilbene that is positioned very deep into the active site, which would explain its luminescence and why 3CFE does not exhibit the same phenomenon. This finding also shows that the luminescence of 3CFB is unique to itself, as this quality is not shared with related proteins. This protein does exhibit some fluorescence; however, it is significantly dimmer than the light produced due to its luminescence. This can be observed at temperatures below 240 degrees Kelvin, as only stilbene fluorescence is seen at temperatures that low. The luminescence of this protein is exhibited at a greater degree at higher temperatures as opposed to lower ones, so the elicitation of this phenomenon should be done at relatively high temperatures as compared to fluorescent phenomena.<ref name="science" /> |
[[Image:trp103.png|200px|right]] | [[Image:trp103.png|200px|right]] | ||
| Line 38: | Line 38: | ||
== Modern Implications == | == Modern Implications == | ||
| - | The mechanism in which this protein emits light is similar to the mechanism used in modern day LEDs, which eludes to the possibility of a biological LED. While it is unlikely to find a true LED occurring naturally, the existence of this protein suggests that bioluminescent marine organisms could have utilized systems similar to an LED in order to give off light. The existence of this protein also suggests the possibility of genetically engineering organisms to exhibit this sort of luminesence. The duration of the light 3CFB emits makes it incredibly useful in biosensing. It can be used in methods such as ELISA assays as an antibody. After UV light exposure, if the desired ligand to be bound to 3CFB is present, the wells which contain the desired compound would glow. The duration and brightness of this glow means that it is an incredibly sensitive qualitative test. It can also be used in sensing mercury, the evaluation of catalysts, sensing cysteine residues on viral protein coats, and in other biosensor applications. In the future, there is hope of developing similarly luminescent | + | The mechanism in which this protein emits light is similar to the mechanism used in modern day LEDs, which eludes to the possibility of a biological LED. While it is unlikely to find a true LED occurring naturally, the existence of this protein suggests that bioluminescent marine organisms could have utilized systems similar to an LED in order to give off light. The existence of this protein also suggests the possibility of genetically engineering organisms to exhibit this sort of luminesence. The duration of the light 3CFB emits makes it incredibly useful in biosensing. It can be used in methods such as ELISA assays as an antibody. After UV light exposure, if the desired ligand to be bound to 3CFB is present, the wells which contain the desired compound would glow. The duration and brightness of this glow means that it is an incredibly sensitive qualitative test. It can also be used in sensing mercury, the evaluation of catalysts, sensing cysteine residues on viral protein coats, and in other biosensor applications. In the future, there is hope of developing similarly luminescent protein-ligand complexes.<ref name="news">Castelvecchi, D. (2013, September 23). True Blue: Electron jumps make protein shine like an LED. Retrieved from https://www.sciencenews.org/article/true-blue-electron-jumps-make-protein-shine-led</ref> |
== References == | == References == | ||
Revision as of 02:00, 2 May 2019
| |||||||||||