Sandbox Reserved 1546
From Proteopedia
| Line 25: | Line 25: | ||
<table><tr><td colspan='2'></td></tr> | <table><tr><td colspan='2'></td></tr> | ||
| - | <tr id='homosapiens'><td class="sblockLbl"><b>Found in These Organisms:</b></td>< | + | <tr id='homosapiens'><td class="sblockLbl"><b>Found in These Organisms:</b></td><span class="plainlinks">''Homo sapiens''</span></td></tr> |
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Number of Ligands:]]</b></td><td class="sblockDat">2 - <scene name='pdbligand=BCO:BUTYRYL+COENZYME+A'>Butyrul CoEnzyme A</scene> & <scene name='pdbligand=EDO:1,2-ETHANEDIOL'>1,2-Ethanediol</scene></td></tr> | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Number of Ligands:]]</b></td><td class="sblockDat">2 - <scene name='pdbligand=BCO:BUTYRYL+COENZYME+A'>Butyrul CoEnzyme A</scene> & <scene name='pdbligand=EDO:1,2-ETHANEDIOL'>1,2-Ethanediol</scene></td></tr> | ||
Revision as of 00:26, 6 May 2019
| This Sandbox is Reserved from May 28 through July 01, 2019 for use in the course Advanced Biochemistry BCHM 4100 taught by Tom Gluick at the Georgia Gwinnett College. This reservation includes Sandbox Reserved 1544 through Sandbox Reserved 1555. |
To get started:
More help: Help:Editing |
Contents |
5H86: HUMAN GCN-5 BOUND TO BUTYRYL-COA
This project was completed by Marianne Javier, Karima MuhummadPoe, and Makayla Yang for Dr. Gluick's Spring 2019 BIOL4100K-01 class at Georgia Gwinnett College.
Project Purpose: We chose this molecule due to its role as a histone acetyltransferase and its role in transcription regulation [1]. It has a harder time having successful enzymatic activity if the acyl-chain is too long which is the purpose of studying the molecule [1]. These researchers made two different acyl-CoA molecules, propionyl-CoA and butyryl-CoA [1]. We are looking at the butyryl-CoA which has a conformation that obstructs the lysine from binding. We chose this molecule to see how Gcn5L2 chooses which acyl-chain donor to have the highest enzymatic activity.
Function
In order to better understand the function of this 5h86 enzyme, we have to understand the basic processes of Fatty Acid Degradation. Fatty Acid Degradation is the procedure that fatty acids go through to be broken down into their metabolites and it takes place in the mitochondrial matrix [2]. Intermediates in the fatty acid breakdown are covalently attached to the sulfhydryl group of coenzyme A [2].
Fatty Acid Degradation happens in three steps:
1. Lipolysis and release from adipose tissue: In the initial steps of degradation, fatty acids are stored in the adipocytes [2]. The breakdown of adipocytes is called lipolysis where they are then released into the bloodstream to circulate through the body [2].
2. Activation and transport into the mitochondria: The mitochondria is where fatty acid oxidation occurs which activates fatty acids to be carried to the mitochondria [2]. The enzyme responsible for the catalysis of this step is Fatty-Acyl Coa Synthetase [2]. Malonyl ACP is the activated donor of two carbon units in the elongation step which is operated by the release of CO2 [2].
3. Β-oxidation: Once inside the mitochodria, five steps occur: 1) Activation by ATP, 2) Oxidation by FAD, 3) Hydration, 4) Oxidation by NAD+, and 5) Thyolysis [2]. The final product is Acetyl-CoA which is now able to be able to enter the TCA cycle [2].
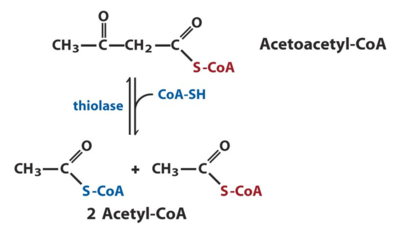
One of the enzymes that have a key role in fatty acid degradation and that is also our enzyme of study is known as acyl-CoA acetyltransferase or thiolase. Thiolases are enzymes that have key roles in biochemical pathways and can either be degrative or biosynthetic [3]. Degrative thiolases such as 3-ketoacyl-CoA thiolase, plays a role in fatty-acid β-oxidation in peroxisomes and mitochondria and ketone body metabolism in mitochondria, whereas biosynthetic thiolases, like acetoacetyl-CoA thiolase, catalyzes Acetoacetyl-CoA to two Acetyl-CoAs [3].
The specific acetyltransferase we are particularly interested in is known as 5h86 which is a Human Gcn5 bound to butyryl-CoA [1]. Gcn5 is a conserved acetyltransferase that regulates transcription by acetylating the N-terminal tails of histones [1]. To better understand how 5h86 is related to fatty acid degradation, we have to understand how they operate as a histone acetyltransferase (HATs). HATs are enzymes that acetylate conserved lysine amino acids on histone proteins [1]. This occurs by transferring an acetyl group from acetyl-CoA to form ε-N-acetyllysine [1]. When DNA is wrapped around histones, an acetyl group is transferred to the histones, allowing genes to be turned on and off [1]. In conclusion, histone acetylation contribute to the increase of gene expression [1].
HATs also have a role in transcription regulation and are regulated through phosphorylation [4]. For example, the HAT activity of the CREB-binding protein (CBP) is stimulated by the phosphorylation of a cyclin E/cyclin-dependent kinase 2 [4]. HATs also participate in the genome-wide yield of acetyl groups on histones [4]. Some HATs also target specific promoters through their physical interaction with sequence-specific transcription factors, sectionally modifying histones or transcription components to regulate gene transcription [4].
In all, the function of the 5H68 Human Gcn5 acetyltransferase can be represented through histone acetylation to assist in the activation of transcription [4]. Histone acetylation functions as the main switch that allows the interchange between permissive and repressive chromatin domains during transcription [5]. The histone acetylation-dependent control of gene expression has mechanisms underlying a direct effect on the solidity of nucleosomal arrays and the creation of key sites for the regulatory binding proteins [5]. The enzymes devoted to the addition and removal of acetyl groups are histone acetyltransferases, such as the 5H68 Human Gcn5 acetyltransferase, and deacetylases, in which both enzymes complete acetyl group removal or addition by removing the lysine residues on the N-terminals of histone tails [5].
Structural Highlights & Disease
Personal tools | |||||||||||