We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Connexin
From Proteopedia
(Difference between revisions)
| Line 42: | Line 42: | ||
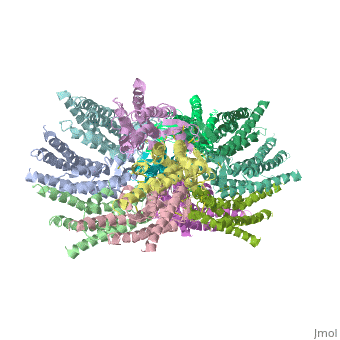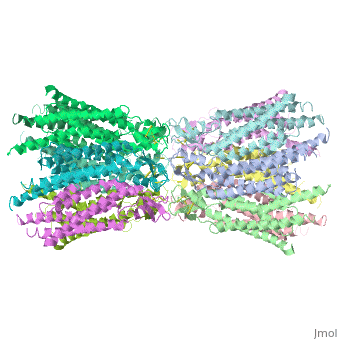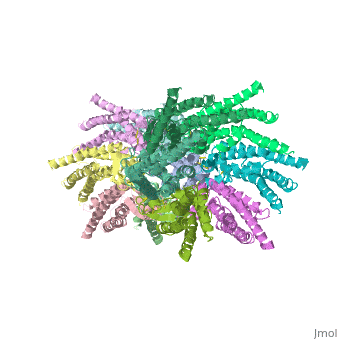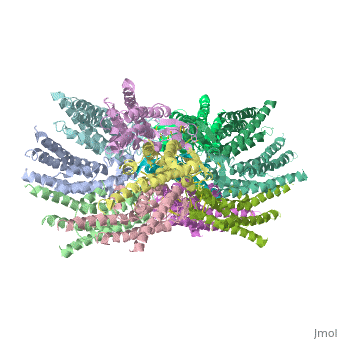
The 3D structure of a mutant human connexin 26 <scene name='70/701426/Mutant_connexin26_-cx26m34a/1'>(Cx26M34A)</scene> channel shows an unexpected density within the vestibule of each hemichannel compared to the <scene name='70/701426/Wild_type_connexin/1'>wild type connexin 26</scene> , which is called a plug <ref name='pdb'/> , That plug was decreased in the the human mutant connexin 26 <scene name='70/701426/Deletion/1'>Cx26del2-7</scene> structure, indicating that the N terminus significantly contributes to form this plug feature. Experiments with this mutant show significantly reduced dye coupling between [http://en.wikipedia.org/wiki/HeLa HeLa cells] transiently expressing Cx26M34A gap junctions. <ref name='pdb'/> | The 3D structure of a mutant human connexin 26 <scene name='70/701426/Mutant_connexin26_-cx26m34a/1'>(Cx26M34A)</scene> channel shows an unexpected density within the vestibule of each hemichannel compared to the <scene name='70/701426/Wild_type_connexin/1'>wild type connexin 26</scene> , which is called a plug <ref name='pdb'/> , That plug was decreased in the the human mutant connexin 26 <scene name='70/701426/Deletion/1'>Cx26del2-7</scene> structure, indicating that the N terminus significantly contributes to form this plug feature. Experiments with this mutant show significantly reduced dye coupling between [http://en.wikipedia.org/wiki/HeLa HeLa cells] transiently expressing Cx26M34A gap junctions. <ref name='pdb'/> | ||
Functional analysis of the Cx26M34A channels revealed that these channels are predominantly closed, with the residual electrical conductance showing normal voltage gating. N-terminal deletion mutants with and without the M34A mutation showed no electrical activity in paired Xenopus oocytes and significantly decreased dye permeability in HeLa cells. Comparing this closed structure with the published X-ray structure of wild-type Cx26, which is proposed to be in an open state, revealed a radial outward shift in the transmembrane helices in the closed state, presumably to accommodate the N-terminal plug occluding the pore. Because both Cx26del2-7 and Cx26M34Adel2-7 channels are closed, the N terminus appears to have a prominent role in stabilizing the open configuration. <ref name='pdb'/> | Functional analysis of the Cx26M34A channels revealed that these channels are predominantly closed, with the residual electrical conductance showing normal voltage gating. N-terminal deletion mutants with and without the M34A mutation showed no electrical activity in paired Xenopus oocytes and significantly decreased dye permeability in HeLa cells. Comparing this closed structure with the published X-ray structure of wild-type Cx26, which is proposed to be in an open state, revealed a radial outward shift in the transmembrane helices in the closed state, presumably to accommodate the N-terminal plug occluding the pore. Because both Cx26del2-7 and Cx26M34Adel2-7 channels are closed, the N terminus appears to have a prominent role in stabilizing the open configuration. <ref name='pdb'/> | ||
| + | |||
| + | =3D structures of connexin= | ||
| + | [[Connexin 3D structure]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
=3D structures of connexin= | =3D structures of connexin= | ||
| Line 51: | Line 55: | ||
**[[2zw3]] – hCX26 – human<br /> | **[[2zw3]] – hCX26 – human<br /> | ||
| - | **[[5er7]], [[5era]] – hCX26 (mutant) <br /> | + | **[[5er7]], [[5era]], [[5kjg]] – hCX26 (mutant) <br /> |
**[[3iz1]], [[3iz2]] – hCX26 (mutant) - CryoEM<br /> | **[[3iz1]], [[3iz2]] – hCX26 (mutant) - CryoEM<br /> | ||
| Line 71: | Line 75: | ||
**[[3shw]] – hCX45 C-terminal peptide + tight junction protein<br /> | **[[3shw]] – hCX45 C-terminal peptide + tight junction protein<br /> | ||
| + | *Connexin 46 or gap junction α-3 protein | ||
| + | |||
| + | **[[6mhq]] – sCX46 – sheep – Cryo EM<br /> | ||
| + | |||
| + | *Connexin 50 or gap junction α-8 protein | ||
| + | |||
| + | **[[6mhy]] – sCX50 – Cryo EM<br /> | ||
}} | }} | ||
Revision as of 09:37, 15 May 2019
| |||||||||||
3D structures of connexin
Updated on 15-May-2019
References
- ↑ Zonta F, Buratto D, Cassini C, Bortolozzi M, Mammano F. Molecular dynamics simulations highlight structural and functional alterations in deafness-related M34T mutation of connexin 26. Front Physiol. 2014 Mar 4;5:85. doi: 10.3389/fphys.2014.00085. eCollection 2014. PMID:24624091 doi:http://dx.doi.org/10.3389/fphys.2014.00085
- ↑ 2.0 2.1 2.2 2.3 Suga M, Maeda S, Nakagawa S, Yamashita E, Tsukihara T. A description of the structural determination procedures of a gap junction channel at 3.5 A resolution. Acta Crystallogr D Biol Crystallogr. 2009 Aug;65(Pt 8):758-66. Epub 2009, Jul 10. PMID:19622859 doi:http://dx.doi.org/10.1107/S0907444909014711
- ↑ http://en.wikipedia.org/wiki/Connexin
- ↑ Sokolov M, Brownstein Z, Frydman M, Avraham KB. Apparent phenotypic anticipation in autosomal dominant connexin 26 deafness. J Basic Clin Physiol Pharmacol. 2014 Sep;25(3):289-92. doi:, 10.1515/jbcpp-2014-0053. PMID:25153233 doi:http://dx.doi.org/10.1515/jbcpp-2014-0053
- ↑ 5.0 5.1 Ambrosi C, Walker AE, Depriest AD, Cone AC, Lu C, Badger J, Skerrett IM, Sosinsky GE. Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix. PLoS One. 2013 Aug 15;8(8):e70916. doi: 10.1371/journal.pone.0070916. eCollection, 2013. PMID:23967136 doi:http://dx.doi.org/10.1371/journal.pone.0070916
- ↑ Kikuchi T, Adams JC, Miyabe Y, So E, Kobayashi T. Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med Electron Microsc. 2000;33(2):51-6. PMID:11810458 doi:http://dx.doi.org/10.1007/s007950000009
- ↑ 7.0 7.1 7.2 7.3 7.4 Oshima A, Tani K, Toloue MM, Hiroaki Y, Smock A, Inukai S, Cone A, Nicholson BJ, Sosinsky GE, Fujiyoshi Y. Asymmetric Configurations and N-terminal Rearrangements in Connexin26 Gap Junction Channels. J Mol Biol. 2011 Jan 21;405(3):724-35. Epub 2010 Nov 20. PMID:21094651 doi:10.1016/j.jmb.2010.10.032
Proteopedia Page Contributors and Editors (what is this?)
Safaa Salah Hussiesy, Michal Harel, Doaa Naffaa, Jaime Prilusky