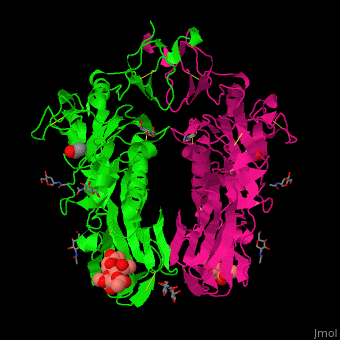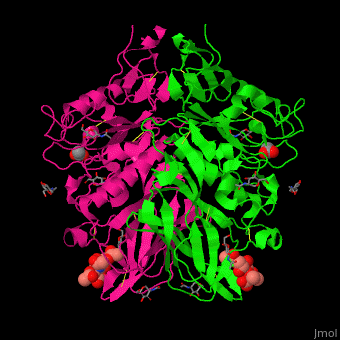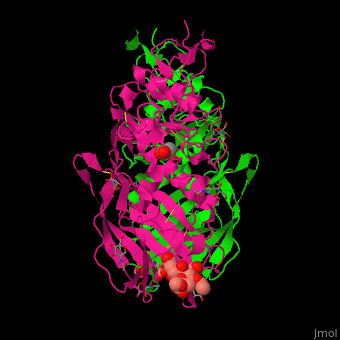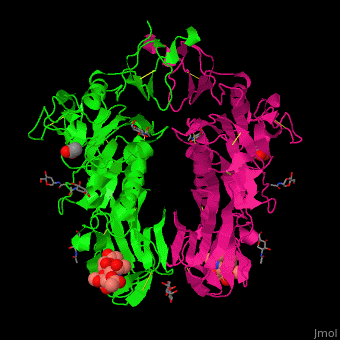Hemagglutinin-esterase
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Structural highlights == | == Structural highlights == | ||
| - | The biological assmebly of HE is a <scene name='48/484851/Cv/ | + | The biological assmebly of HE is a <scene name='48/484851/Cv/7'>dimer</scene> and each monomer contains <scene name='48/484851/Cv/8'>3 domains: the membrane fusion domain, the esterase domain and the receptor-binding domain</scene>. The receptor binding site of HE is located at five exposed surface loops. <scene name='48/484851/Cv/9'>Acetylated sialic acid binding site</scene>. HE contains a <scene name='48/484851/Cv/10'>conserved metal-binding site</scene><ref>PMID:22291594</ref>. |
</StructureSection> | </StructureSection> | ||
==3D structures of hemagglutinin-esterase== | ==3D structures of hemagglutinin-esterase== | ||
Revision as of 13:12, 15 May 2019
| |||||||||||
3D structures of hemagglutinin-esterase
Updated on 15-May-2019
References
- ↑ de Groot RJ. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj J. 2006 Feb;23(1-2):59-72. PMID:16575523 doi:http://dx.doi.org/10.1007/s10719-006-5438-8
- ↑ Langereis MA, Zeng Q, Heesters BA, Huizinga EG, de Groot RJ. The murine coronavirus hemagglutinin-esterase receptor-binding site: a major shift in ligand specificity through modest changes in architecture. PLoS Pathog. 2012 Jan;8(1):e1002492. doi: 10.1371/journal.ppat.1002492. Epub 2012, Jan 26. PMID:22291594 doi:http://dx.doi.org/10.1371/journal.ppat.1002492