User:Evžen Wybitul/Sandbox 1
From Proteopedia
| Line 1: | Line 1: | ||
| - | [[Voltage-gated calcium channel Cav1.1 complex|Cav1.1]] is a protein from the family of Cav1 voltage dependent Ca channels, all of which link the depolarization of the cytoplasmic membrane to the signaling pathways in the cell. In particular, Cav1.1, together with other ion channels and proteins, mediates signals from a neuronal cell to a muscle cell, thus playing a key role in the regulatory processes of muscular activity. A point mutation in one of its subunits is connected to the HOKPP disease. | + | [[Image:Example.jpg]][[Voltage-gated calcium channel Cav1.1 complex|Cav1.1]] is a protein from the family of Cav1 voltage dependent Ca channels, all of which link the depolarization of the cytoplasmic membrane to the signaling pathways in the cell. In particular, Cav1.1, together with other ion channels and proteins, mediates signals from a neuronal cell to a muscle cell, thus playing a key role in the regulatory processes of muscular activity. A point mutation in one of its subunits is connected to the HOKPP disease. |
==Voltage-gated calcium channel Cav1.1 complex== | ==Voltage-gated calcium channel Cav1.1 complex== | ||
<StructureSection load='5GJV' size='340' side='right' caption='Mammalian voltage-gated calcium channel Cav1.1 complex' scene=''> | <StructureSection load='5GJV' size='340' side='right' caption='Mammalian voltage-gated calcium channel Cav1.1 complex' scene=''> | ||
This transmembrane protein consists of five subunits (α1, α2/δ, β, γ). α1 subunit forms an ion-conducting pore and includes receptors for dihydropyridin, while the rest regulates the function of the channel itself. Every subunit is composed from four transmembrane domains (DI–DIV), each of which has six transmembrane α helices (S1–S6). The S1–S4 form a voltage-sensitive domain, and by the S5 and S6 a single pore domain is formed. | This transmembrane protein consists of five subunits (α1, α2/δ, β, γ). α1 subunit forms an ion-conducting pore and includes receptors for dihydropyridin, while the rest regulates the function of the channel itself. Every subunit is composed from four transmembrane domains (DI–DIV), each of which has six transmembrane α helices (S1–S6). The S1–S4 form a voltage-sensitive domain, and by the S5 and S6 a single pore domain is formed. | ||
| + | |||
| + | Only some domains of the human Cav1.1 were determined, while the whole structure of rabbit Cav1.1 was ascertained in 2016. The rabbit version of this protein has 93% sequence identity with the human one, which is why we eventually opted to use the structure of rabbit Cav1.1 instead, which you can now see on the right. | ||
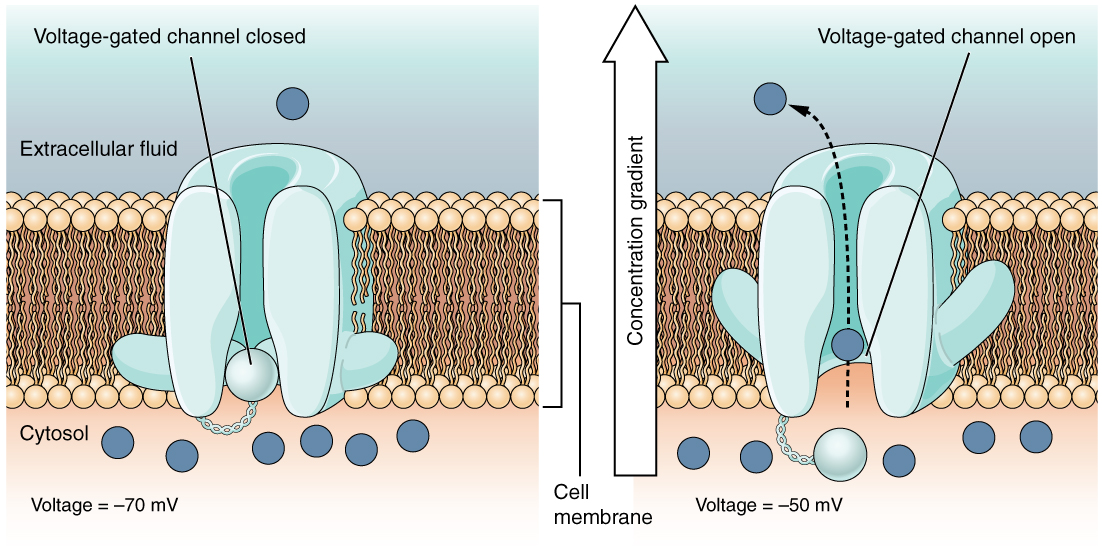
=== Closed state === | === Closed state === | ||
| - | The S4 helix is being pulled inwards by the negative resting potential. The outermost arginine interacts with residues in other helices and so | + | The S4 helix is being pulled inwards by the negative resting potential of the membrane. The outermost arginine interacts with residues in other helices and so a voltage-sensing domain is formed. |
=== Open state === | === Open state === | ||
| Line 67: | Line 69: | ||
In closed state, a new permeation pathway can appear when arginine in position 1 on the S4 helix is mutated to an uncharged residue such as serine or glycine. This difference is caused by the movement of the S4 helix when the channel opens and closes; the Arg 1 pore is blocked by Arg 3 when the channel is open and opened when the channel is closed. <ref name="orig"/> | In closed state, a new permeation pathway can appear when arginine in position 1 on the S4 helix is mutated to an uncharged residue such as serine or glycine. This difference is caused by the movement of the S4 helix when the channel opens and closes; the Arg 1 pore is blocked by Arg 3 when the channel is open and opened when the channel is closed. <ref name="orig"/> | ||
| - | + | [[Image:Example.jpg]] | |
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 13:13, 17 May 2019
 Cav1.1 is a protein from the family of Cav1 voltage dependent Ca channels, all of which link the depolarization of the cytoplasmic membrane to the signaling pathways in the cell. In particular, Cav1.1, together with other ion channels and proteins, mediates signals from a neuronal cell to a muscle cell, thus playing a key role in the regulatory processes of muscular activity. A point mutation in one of its subunits is connected to the HOKPP disease.
Cav1.1 is a protein from the family of Cav1 voltage dependent Ca channels, all of which link the depolarization of the cytoplasmic membrane to the signaling pathways in the cell. In particular, Cav1.1, together with other ion channels and proteins, mediates signals from a neuronal cell to a muscle cell, thus playing a key role in the regulatory processes of muscular activity. A point mutation in one of its subunits is connected to the HOKPP disease.
Contents |
Voltage-gated calcium channel Cav1.1 complex
| |||||||||||
Function
After the release of neurotransmitter, usually acetylcholine, into the synaptic cleft, and its subsequent reaction with the corresponding receptors on a muscle cell, the voltage dependent Na channels are opened, resulting in a depolarization of the usually slightly negatively charged cytoplasmic membrane.[1] This in turn activates the voltage dependent Cav1.1 channels located in T-tubules, which are mechanically connected to RyR1 receptors on the sarcoplasmic reticulum; [2] this activation results in a conformational change which allosterically activates the RyR1. Through it the Ca release channels present on the same organelle are also activated. [3] This sudden release of Ca causes muscle contraction.
Membrane hyperpolarization
Typically, the concentration of sodium in extracellular space is many times higher than the concentration of potassium, and vice versa in the cytoplasm. This concentration gradient is constantly maintained by ATP sodium-potassium pumps and is the driving force behind the Na influx in the process of membrane depolarization, which is crucial for the correct function of muscle tissue, as explained above.
If we lower the concentration of K in the extracellular space, the concentration gradient gets stronger and consequently some amount of potassium moves from the cell. When the potassium cations exit the cell, the membrane charge becomes more negative and the polarization gap between extracellular space and the membrane further widens through a process called hyperpolarization.
However, if we continue to lower the concentration of extracellular potassium, an interesting phenomenon can be observed --- instead of becoming more hyperpolarized, the membrane suddenly depolarizes. At fault are the leak inward currents: nonselective pathways into the cell whose activity is driven mainly by physical forces, that are in stark contrast with selective rectifier K+-channels, which try to restore the former membrane potential. [4] When the concentration of extracellular K crosses a certain threshold, the leaks outweigh the rectifiers and a new equilibrium is found, with resting membrane potential absolute value being much lower than it was before. [5] The exact value of the threshold varies, but depends heavily on the number of leaks in the cell membrane and on the number and effectivity of rectifiers.
Disease — Hypokalemic periodic paralysis
Hypokalemic periodic paralysis is a condition that causes episodes of extreme muscle weakness. People with hypokalemic periodic paralysis have reduced levels of potassium in their blood (hypokalemia) during episodes of muscle weakness. These episodes mostly involve a temporary inability to move limbs [6]. Episodes may cause severe weakness that usually lasts from hours to days. Episodes can occur without any reason or can be triggered by factors such as exercising, a viral illness, certain medications or a large, carbohydrate-rich meal [7]. Although affected individuals usually gain their muscle strength back between episodes, repeated episodes can lead to persistent muscle weakness later in life.
Prevalence
Hypokalemic periodic paralysis typically begins in childhood or adolescence. Although its exact prevalence is unknown, the disease estimately affects 1 in 100,000 people. Men experience symptoms of this condition more often than women.
Heredity
This condition is inherited in an autosomal dominant pattern, which means one mutated copy of the gene in each cell is sufficient for a person to be affected.
In most cases, the mutated gene is inherited from an affected parent. However, inheritance from a parent may sometimes be masked by a parent not having symptoms, as not all people with this mutation have symptoms of the condition. This phenomenon is called reduced penetrance.
The disease-causing mutation may occur for the first time in a person with no family history of this disease. This is called a de novo mutation. The proportion of cases caused by a de novo mutation is unknown.
Diagnosis
A diagnosis of Hypokalemic periodic paralysis is based on:
- History of episodes of paralysis,
- Family history associted with HOKPP.
- Low levels of potassium during attacks, but not between attacks.
- Identification of typical triggers [8].
The diagnosis cannot be established by clinical findings alone without a known family history of the condition. Which means, that the condition in the people in which the mutation occurs de novo often stays undiagnosed. Modern medicine uses Various types of tests including blood tests and urine tests.
Treatment
The most common treatment in periodic paralysis is generally considered to be acetazolamide thus there is no standardised treatment regimen and no consensus as to when to start treatment. It is not yet clear if acetazolamide treatment prevents the permanent weakness that may occur by time.
There is an evidence of two small studies demonstrating an improvement of muscle strength with pinacidil and acetazolamide.
HOKPP and the structure of Cav1.1
In patients with HOKPP, muscle weakness emerges in conjunction with hypokalemia. Thus we can deduce that a pathological depolarization occurs on the membrane of the muscle cell, due to which the muscles are rendered unusable.
As described above, this depolarization occurs even in healthy cells when the potassium levels drop too low, but the threshold can be lowered by blocking the inward rectifier K+ current and by enhancing the depolarizing leak current. Both of these would make the muscle cell more sensitive to extracellular potassium levels and could be possibly involved in the mechanism of HOKPP. However, as there are no HOKPP patients with mutated K+ rectifier channels, it can be assumed that only the strenghtening of the leak currents is at play. [9]
In closed state, a new permeation pathway can appear when arginine in position 1 on the S4 helix is mutated to an uncharged residue such as serine or glycine. This difference is caused by the movement of the S4 helix when the channel opens and closes; the Arg 1 pore is blocked by Arg 3 when the channel is open and opened when the channel is closed. [4]
References
- ↑ Alberts, Bruce. "Neuromuscular Transmission Involves the Sequential Activation of Five Different Sets of Ion Channels." Molecular biology of the cell. Johanneshov: MTM, 2017. 632-33.
- ↑ Kugler G, Weiss RG, Flucher BE, Grabner M. Structural requirements of the dihydropyridine receptor alpha1S II-III loop for skeletal-type excitation-contraction coupling. J Biol Chem. 2004 Feb 6;279(6):4721-8. doi: 10.1074/jbc.M307538200. Epub 2003 Nov, 18. PMID:14627713 doi:http://dx.doi.org/10.1074/jbc.M307538200
- ↑ Proenza C, O'Brien J, Nakai J, Mukherjee S, Allen PD, Beam KG. Identification of a region of RyR1 that participates in allosteric coupling with the alpha(1S) (Ca(V)1.1) II-III loop. J Biol Chem. 2002 Feb 22;277(8):6530-5. doi: 10.1074/jbc.M106471200. Epub 2001, Nov 28. PMID:11726651 doi:http://dx.doi.org/10.1074/jbc.M106471200
- ↑ 4.0 4.1 Striessnig J, Bolz HJ, Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch. 2010 Jul;460(2):361-74. doi: 10.1007/s00424-010-0800-x. Epub 2010 , Mar 7. PMID:20213496 doi:http://dx.doi.org/10.1007/s00424-010-0800-x
- ↑ Struyk AF, Cannon SC. Paradoxical depolarization of BA2+- treated muscle exposed to low extracellular K+: insights into resting potential abnormalities in hypokalemic paralysis. Muscle Nerve. 2008 Mar;37(3):326-37. doi: 10.1002/mus.20928. PMID:18041053 doi:http://dx.doi.org/10.1002/mus.20928
- ↑ Hypokalemic periodic paralysis. Genetics Home Reference. April 2007; http://ghr.nlm.nih.gov/condition=hypokalemicperiodicparalysis.
- ↑ Hypokalemic periodic paralysis. MedlinePlus. October 13, 2015; http://www.nlm.nih.gov/medlineplus/ency/article/000312.htm
- ↑ Savine Vicart, et al. Hypokalemic Periodic Paralysis. GeneReviews. July 31, 2014; https://www.ncbi.nlm.nih.gov/books/NBK1338/
- ↑ Lapie P, Lory P, Fontaine B. Hypokalemic periodic paralysis: an autosomal dominant muscle disorder caused by mutations in a voltage-gated calcium channel. Neuromuscul Disord. 1997 Jun;7(4):234-40. doi: 10.1016/s0960-8966(97)00435-5. PMID:9196905 doi:http://dx.doi.org/10.1016/s0960-8966(97)00435-5
