We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Victor Reverte/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Function == | == Function == | ||
| - | '''MCU''' is a highly selective uniporter that allows calcium entry into the mitochondrial matrix. In metazoa, it belongs to a protein complex entitled '''Mitochondrial Calcium Uniporter Complex (MCUC)''' along with '''EMRE''', '''MICU1''' and '''MICU2''', which are respectively proposed as a regulator and gatekeepers of the complex. | + | '''MCU''' is a highly selective uniporter that allows calcium entry into the mitochondrial matrix<ref>DOI 10.1038/ncb2868</ref>. In metazoa, it belongs to a protein complex entitled '''Mitochondrial Calcium Uniporter Complex (MCUC)''' along with '''EMRE''', '''MICU1''' and '''MICU2''', which are respectively proposed as a regulator and gatekeepers of the complex<ref>DOI 10.1161/CIRCRESAHA.116.305484</ref>. |
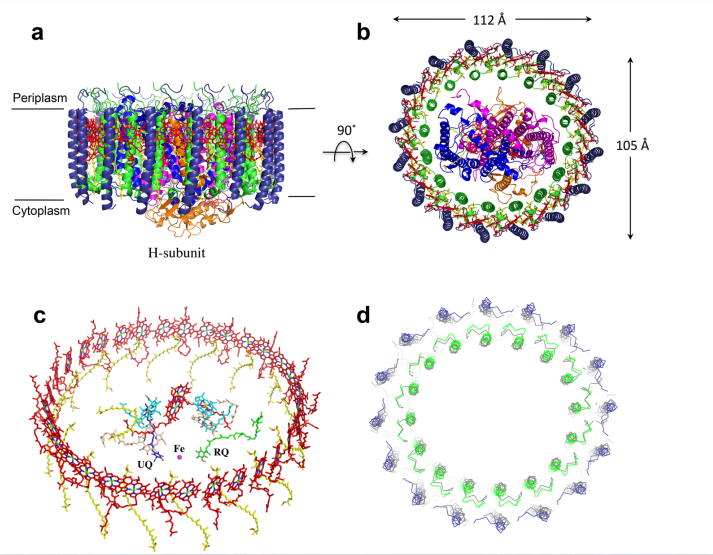
== Structure == | == Structure == | ||
| - | Being assembled as a tetramer, '''MCU''' monomers posses 351 amino acid residues. Each subunit can be divided into four structural domains, them being '''N-terminal domain (NTD)''', '''linker helix domain (LHD)''', '''coiled-coil domain (CCD)''', and '''transmembrane domain (TMD)'''. '''CCD''' and '''TMD''' are the pore-forming subunits, while '''LHD''' links this regions to '''NTD'''. Recently, regulation of the complex and dimerization of two tetrameres were reported as functions of '''NTD'''. | + | Being assembled as a tetramer, '''MCU''' monomers posses 351 amino acid residues. Each subunit can be divided into four structural domains, them being '''N-terminal domain (NTD)''', '''linker helix domain (LHD)''', '''coiled-coil domain (CCD)''', and '''transmembrane domain (TMD)'''. '''CCD''' and '''TMD''' are the pore-forming subunits, while '''LHD''' links this regions to '''NTD'''. Recently, regulation of the complex and dimerization of two tetrameres were reported as functions of '''NTD'''<ref>DOI 10.1016/j.chembiol.2016.07.012</ref>. |
| - | To guarantee selectivity, <scene name='81/817978/260wdimep265/1'>260WDIMEP265</scene>, a highly conserved sequence among protein homologues, from each monomer form two filters. The first one, dependent of <scene name='81/817978/D261/1'>D261 residues</scene> has a radius of affinity for hydrated calcium. The narrower one, is stabilized by <scene name='81/817978/E264/2'>E264</scene> and its selective for calcium radius. Finally, there’s a second constriction point at the end of the pore, formed by residues <scene name='81/817978/E288_and_v290/1'>E288 and V290</scene> of each monomer, that are involved in a juxtamembrane loop (JML). | + | To guarantee selectivity, <scene name='81/817978/260wdimep265/1'>260WDIMEP265</scene>, a highly conserved sequence among protein homologues, from each monomer form two filters. The first one, dependent of <scene name='81/817978/D261/1'>D261 residues</scene> has a radius of affinity for hydrated calcium. The narrower one, is stabilized by <scene name='81/817978/E264/2'>E264</scene> and its selective for calcium radius. Finally, there’s a second constriction point at the end of the pore, formed by residues <scene name='81/817978/E288_and_v290/1'>E288 and V290</scene> of each monomer, that are involved in a juxtamembrane loop (JML)<ref>DOI 10.1016/j.cell.2019.03.050</ref>. |
[[Image:Structure.png]] | [[Image:Structure.png]] | ||
| Line 18: | Line 18: | ||
== Disease == | == Disease == | ||
| - | While having a crucial role in calcium homeostasis, mitochondrial calcium uptake was already reported as protective to neuron excitotoxicity and liver ischemia-reperfusion damage. Therefore, mutations and dysfunction of '''MCU''' could aggravate these kinds of insult. Also, MCU could be protective to skeletal muscle age related atrophy, as suggested by it’s increase expression upon exercise stimuli. | + | While having a crucial role in calcium homeostasis, mitochondrial calcium uptake was already reported as protective to neuron excitotoxicity and liver ischemia-reperfusion damage<ref>DOI 10.1111/acel.12527</ref>. Therefore, mutations and dysfunction of '''MCU''' could aggravate these kinds of insult. Also, MCU could be protective to skeletal muscle age related atrophy, as suggested by it’s increase expression upon exercise stimuli<ref>DOI 10.1016/j.freeradbiomed.2017.06.013</ref>. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 13:13, 10 June 2019
Mitochondrial Calcium Uniporter (MCU)
| |||||||||||
References
- ↑ Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003 Jul;4(7):517-29. PMID:12838335 doi:10.1038/nrm1155
- ↑ Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012 Sep;13(9):566-78. doi: 10.1038/nrm3412. Epub 2012 Aug, 1. PMID:22850819 doi:http://dx.doi.org/10.1038/nrm3412
- ↑ Chaudhuri D, Sancak Y, Mootha VK, Clapham DE. MCU encodes the pore conducting mitochondrial calcium currents. Elife. 2013 Jun 4;2:e00704. doi: 10.7554/eLife.00704. PMID:23755363 doi:http://dx.doi.org/10.7554/eLife.00704
- ↑ Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013 Dec;15(12):1464-72. doi: 10.1038/ncb2868. Epub 2013 Nov 10. PMID:24212091 doi:http://dx.doi.org/10.1038/ncb2868
- ↑ Finkel T, Menazza S, Holmstrom KM, Parks RJ, Liu J, Sun J, Liu J, Pan X, Murphy E. The ins and outs of mitochondrial calcium. Circ Res. 2015 May 22;116(11):1810-9. doi: 10.1161/CIRCRESAHA.116.305484. PMID:25999421 doi:http://dx.doi.org/10.1161/CIRCRESAHA.116.305484
- ↑ Lee SK, Shanmughapriya S, Mok MC, Dong Z, Tomar D, Carvalho E, Rajan S, Junop MS, Madesh M, Stathopulos PB. Structural Insights into Mitochondrial Calcium Uniporter Regulation by Divalent Cations. Cell Chem Biol. 2016 Aug 24. pii: S2451-9456(16)30242-2. doi:, 10.1016/j.chembiol.2016.07.012. PMID:27569754 doi:http://dx.doi.org/10.1016/j.chembiol.2016.07.012
- ↑ Wang Y, Nguyen NX, She J, Zeng W, Yang Y, Bai XC, Jiang Y. Structural Mechanism of EMRE-Dependent Gating of the Human Mitochondrial Calcium Uniporter. Cell. 2019 May 16;177(5):1252-1261.e13. doi: 10.1016/j.cell.2019.03.050. Epub, 2019 May 9. PMID:31080062 doi:http://dx.doi.org/10.1016/j.cell.2019.03.050
- ↑ Wang Y, Nguyen NX, She J, Zeng W, Yang Y, Bai XC, Jiang Y. Structural Mechanism of EMRE-Dependent Gating of the Human Mitochondrial Calcium Uniporter. Cell. 2019 May 16;177(5):1252-1261.e13. doi: 10.1016/j.cell.2019.03.050. Epub, 2019 May 9. PMID:31080062 doi:http://dx.doi.org/10.1016/j.cell.2019.03.050
- ↑ Amigo I, Menezes-Filho SL, Luevano-Martinez LA, Chausse B, Kowaltowski AJ. Caloric restriction increases brain mitochondrial calcium retention capacity and protects against excitotoxicity. Aging Cell. 2017 Feb;16(1):73-81. doi: 10.1111/acel.12527. Epub 2016 Sep 13. PMID:27619151 doi:http://dx.doi.org/10.1111/acel.12527
- ↑ Menezes-Filho SL, Amigo I, Prado FM, Ferreira NC, Koike MK, Pinto IFD, Miyamoto S, Montero EFS, Medeiros MHG, Kowaltowski AJ. Caloric restriction protects livers from ischemia/reperfusion damage by preventing Ca(2+)-induced mitochondrial permeability transition. Free Radic Biol Med. 2017 Sep;110:219-227. doi:, 10.1016/j.freeradbiomed.2017.06.013. Epub 2017 Jun 19. PMID:28642067 doi:http://dx.doi.org/10.1016/j.freeradbiomed.2017.06.013