User:Jonathan Cardoso C. Vieira/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
== Polymerization mechanism == | == Polymerization mechanism == | ||
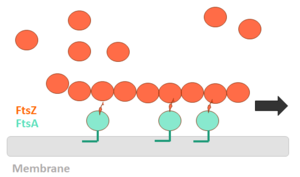
In order for polymerization of the monomers of FtsZ to occur, there is a need for interaction of the N-terminal nucleotide binding domain with the C-terminal domain of another monomer. The formation of a complete GTP hydrolysis site depends on the positioning of acid residues of the T7 loop at the nucleotide binding site of the prior monomer in the polymer, explaining why the GTPase activity of FtsZ only occurs when the protein is in the polymer form. The fact that FtsZ GTPase activity interferes with the dynamics of its autoassociation and the morphology of its polymers, as well as with microtubules and actin, opens the possibility that FtsZ can also present dynamic treadmilling or instability. Dynamic FtsZ beams were observed in vitro and protofilaments exhibiting treadmilling dependent on FtsA protein. | In order for polymerization of the monomers of FtsZ to occur, there is a need for interaction of the N-terminal nucleotide binding domain with the C-terminal domain of another monomer. The formation of a complete GTP hydrolysis site depends on the positioning of acid residues of the T7 loop at the nucleotide binding site of the prior monomer in the polymer, explaining why the GTPase activity of FtsZ only occurs when the protein is in the polymer form. The fact that FtsZ GTPase activity interferes with the dynamics of its autoassociation and the morphology of its polymers, as well as with microtubules and actin, opens the possibility that FtsZ can also present dynamic treadmilling or instability. Dynamic FtsZ beams were observed in vitro and protofilaments exhibiting treadmilling dependent on FtsA protein. | ||
| + | [[Image:Polimerization FtsZ.png | thumb | left | alt=Puzzle globe| FtsZ polimerization mechanism | 300 px]] | ||
The binding of GTP promotes the longitudinal association of the monomers forming protofilaments, and the hydrolysis of the nucleotide leads to depolymerization and consequent disorganization of the protofilament. FtsZ protofilaments have a strong tendency to associate further to form multi-stranded polymers. The tendency of FtsZ filaments to form lateral interactions in greatly increased by the presence of cations such the Ca2+. | The binding of GTP promotes the longitudinal association of the monomers forming protofilaments, and the hydrolysis of the nucleotide leads to depolymerization and consequent disorganization of the protofilament. FtsZ protofilaments have a strong tendency to associate further to form multi-stranded polymers. The tendency of FtsZ filaments to form lateral interactions in greatly increased by the presence of cations such the Ca2+. | ||
However, there is still controversy regarding the mechanism of polymerization of FtsZ and details of the process are being elucidated. What is currently in place is that loop motion between the H6-H7 helices away from the nucleotide cavity (downward movement of the H7 helix, and 23-degree rotation of the C-terminal domain relative to the N-terminal), creates a groove between the C-terminal domain and the H7 helix that does not exist in the structures described above. This conformation promotes the insertion of the T7 loop in the active site of another monomer, which in the presence of divalent cation would stabilize a dimeric interface with more extensive contacts leading to polymerization. Therefore, the "assembly switch" would be the conversion of a monomer into the closed conformation (without the groove between H7 and C-terminal) to the open conformation (with the groove between H7 and the C-terminus), the latter competent for polymerization. | However, there is still controversy regarding the mechanism of polymerization of FtsZ and details of the process are being elucidated. What is currently in place is that loop motion between the H6-H7 helices away from the nucleotide cavity (downward movement of the H7 helix, and 23-degree rotation of the C-terminal domain relative to the N-terminal), creates a groove between the C-terminal domain and the H7 helix that does not exist in the structures described above. This conformation promotes the insertion of the T7 loop in the active site of another monomer, which in the presence of divalent cation would stabilize a dimeric interface with more extensive contacts leading to polymerization. Therefore, the "assembly switch" would be the conversion of a monomer into the closed conformation (without the groove between H7 and C-terminal) to the open conformation (with the groove between H7 and the C-terminus), the latter competent for polymerization. | ||
Revision as of 17:37, 14 June 2019
| |||||||||||