User:Jonathan Cardoso C. Vieira/Sandbox 1
From Proteopedia
< User:Jonathan Cardoso C. Vieira(Difference between revisions)
| Line 12: | Line 12: | ||
== Polymerization mechanism == | == Polymerization mechanism == | ||
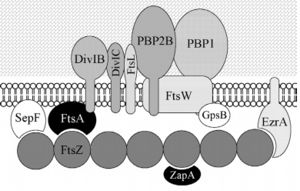
| - | In order for polymerization of the monomers of FtsZ to occur, there is a need for interaction of the N-terminal nucleotide binding domain with the C-terminal domain of another monomer. The formation of a complete GTP hydrolysis site depends on the positioning of acid residues of the T7 loop at the nucleotide binding site of the prior monomer in the polymer, explaining why the GTPase activity of FtsZ only occurs when the protein is in the polymer form. The fact that FtsZ GTPase activity interferes with the dynamics of its autoassociation and the morphology of its polymers, as well as with microtubules and actin, opens the possibility that FtsZ can also present dynamic treadmilling or instability. Dynamic FtsZ beams were observed in vitro and protofilaments exhibiting treadmilling dependent on FtsA protein. | + | In order for polymerization of the monomers of FtsZ to occur, there is a need for interaction of the N-terminal nucleotide binding domain with the C-terminal domain of another monomer. The formation of a complete GTP hydrolysis site depends on the positioning of acid residues of the T7 loop at the nucleotide binding site of the prior monomer in the polymer, explaining why the GTPase activity of FtsZ only occurs when the protein is in the polymer form. <scene name='47/473540/Cv/4'>The GTP binding site</scene> (PDB code [[1w5b]]). Water molecules are shown as red spheres. The fact that FtsZ GTPase activity interferes with the dynamics of its autoassociation and the morphology of its polymers, as well as with microtubules and actin, opens the possibility that FtsZ can also present dynamic treadmilling or instability. Dynamic FtsZ beams were observed in vitro and protofilaments exhibiting treadmilling dependent on FtsA protein. |
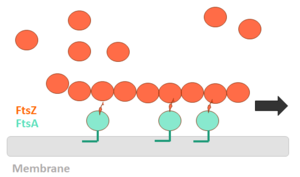
[[Image:Polimerization FtsZ.png | thumb | left | alt=Puzzle globe| FtsZ polimerization mechanism | 300 px]] | [[Image:Polimerization FtsZ.png | thumb | left | alt=Puzzle globe| FtsZ polimerization mechanism | 300 px]] | ||
The binding of GTP promotes the longitudinal association of the monomers forming protofilaments, and the hydrolysis of the nucleotide leads to depolymerization and consequent disorganization of the protofilament. FtsZ protofilaments have a strong tendency to associate further to form multi-stranded polymers. The tendency of FtsZ filaments to form lateral interactions in greatly increased by the presence of cations such the Ca2+. | The binding of GTP promotes the longitudinal association of the monomers forming protofilaments, and the hydrolysis of the nucleotide leads to depolymerization and consequent disorganization of the protofilament. FtsZ protofilaments have a strong tendency to associate further to form multi-stranded polymers. The tendency of FtsZ filaments to form lateral interactions in greatly increased by the presence of cations such the Ca2+. | ||
Current revision
| |||||||||||
References
[1] [2] [3] [4] [5] [6] [7] [8]
- ↑ Bisson-Filho AW, Discola KF, Castellen P, Blasios V, Martins A, Sforca ML, Garcia W, Zeri AC, Erickson HP, Dessen A, Gueiros-Filho FJ. FtsZ filament capping by MciZ, a developmental regulator of bacterial division. Proc Natl Acad Sci U S A. 2015 Apr 6. pii: 201414242. PMID:25848052 doi:http://dx.doi.org/10.1073/pnas.1414242112
- ↑ Wang, X. & Lutkenhaus, J. FtsZ ring: the eubacterial division apparatus conserved in archaebacteria.Mol. Microbiol. 21, 313–319 (1996). Gueiros-Filho, F. J. & Losick, R. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 16, 2544–2556 (2002).
- ↑ Wang, X., Huang, J., Mukherjee, A., Cao, C., and Lutkenhaus, J. (1997). Analysis of the interaction of FtsZ with itself, GTP, and FtsA. J. Bacteriol. 179, 5551–5559.
- ↑ Vaughan S, Wickstead B, Gull K, Addinall SG. Molecular evolution of FtsZ protein sequences encoded within the genomes of archaea, bacteria, and eukaryota. J Mol Evol. 2004 Jan;58(1):19-29. doi: 10.1007/s00239-003-2523-5. PMID:14743312 doi:http://dx.doi.org/10.1007/s00239-003-2523-5
- ↑ Szwedziak P, Wang Q, Bharat TA, Tsim M, Lowe J. Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. Elife. 2014 Dec 9;3:e04601. doi: 10.7554/eLife.04601. PMID:25490152 doi:http://dx.doi.org/10.7554/eLife.04601
- ↑ Huecas, S. et al. Energetics and geometry of FtsZ polymers: nucleated self-assembly of single protofilaments. Biophys. J. 94, 1796–1806 (2008).
- ↑ Lan, G. et al. (2009) Condensation of FtsZ filaments can drive bacterial cell division. Proc. Natl. Acad. Sci. U. S. A. 106, 121–126
- ↑ FILHO, Frederico Gueiros. Cell Division. In: GRAUMANN, Peter L. et al. Bacillus: Cellular and Molecular Biology. Germany: Caister Academic Press, 2017.