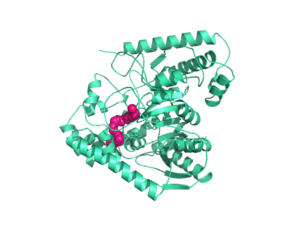
Fatty acid amide hydrolase
From Proteopedia
(Difference between revisions)
(adding BAMBED ref) |
|||
| Line 28: | Line 28: | ||
{{Clear}} | {{Clear}} | ||
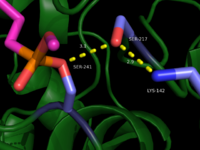
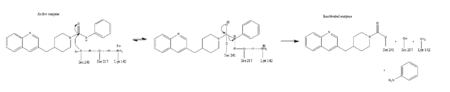
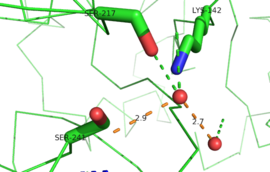
This evidence of convergent evolution between FAAH and other amidase signature enzymes supports the [http://euch6f.chem.emory.edu/burgidunitz.html Bürgi-Dunitz theory]. The Bürgi-Dunitz theory proposes that nucleophiles tend to follow a specific trajectory when attacking a carbonyl, resulting in many enzyme mechanisms having the same angle between an incoming nucleophile and the carbonyl it attacks [http://www.nature.com/nrd/journal/v11/n4/images/nrd3673-f4.jpg (FAAH bound to inhibitor)]. Water molecules in the active sites of enzymes are specifically positioned to force the nucleophile to approach at the exact [http://3.bp.blogspot.com/-NvsQyVPnLIw/UO91-BQTVgI/AAAAAAAAExw/-seGZcjU3DE/s400/burgi-duntz+trajectory.png "Bürgi-Dunitz angle"] of 107°. The determination that FAAH also has water molecules in its active site, helping the nucleophile to attack the amide carbonyl at a specific angle, adds additional support to the Bürgi-Dunitz theory <ref name="3LJ6"/>. | This evidence of convergent evolution between FAAH and other amidase signature enzymes supports the [http://euch6f.chem.emory.edu/burgidunitz.html Bürgi-Dunitz theory]. The Bürgi-Dunitz theory proposes that nucleophiles tend to follow a specific trajectory when attacking a carbonyl, resulting in many enzyme mechanisms having the same angle between an incoming nucleophile and the carbonyl it attacks [http://www.nature.com/nrd/journal/v11/n4/images/nrd3673-f4.jpg (FAAH bound to inhibitor)]. Water molecules in the active sites of enzymes are specifically positioned to force the nucleophile to approach at the exact [http://3.bp.blogspot.com/-NvsQyVPnLIw/UO91-BQTVgI/AAAAAAAAExw/-seGZcjU3DE/s400/burgi-duntz+trajectory.png "Bürgi-Dunitz angle"] of 107°. The determination that FAAH also has water molecules in its active site, helping the nucleophile to attack the amide carbonyl at a specific angle, adds additional support to the Bürgi-Dunitz theory <ref name="3LJ6"/>. | ||
| - | |||
==Applications== | ==Applications== | ||
| Line 35: | Line 34: | ||
{{Clear}} | {{Clear}} | ||
FAAH plays a role in endocannabinoid signaling that has intriguing potential as a drug target. This signaling system consists of endocannabinoid ligands (such as AEA), two G protein-coupled receptors (CB1 and CB2), and the enzymes that synthesize and degrade (such as FAAH) the signaling lipids. Previous research has explored the potential of regulating endocannabinoid signaling through the CB1 and CB2 receptors. However, molecules found to activate these receptors (such as [http://www.ch.ic.ac.uk/vchemlib/mim/bristol/thc/thc_text.htm tetrahydrocannabinol] (THC), the main psychoactive ingredient of [http://en.wikipedia.org/wiki/Cannabis_(drug) marijuana]), while providing the intended pain relief, also produce many undesirable side effects, such as decreased cognition and motor control. On the other hand, research involving FAAH inhibitors has shown that blocking this part of the pathway reduces pain without the unwanted side effects seen through CB1/CB2 activation. Thus, exploring the possibility of using FAAH inhibition to decrease pain relief with minimal side effects could lead to new pain treatment solutions. For example, the [http://drugdiscoveryopinion.com/2009/04/pf-3845-%E2%80%93-a-potent-and-selective-faah-inhibitor/ PF-3845 inhibitor]of FAAH is extremely selective for the enzyme and raises AEA levels for a prolonged duration of time. Therefore, this inhibitor could be explored as a possible pharmaceutical product to target FAAH when extended pain relief is desired <ref name="2WAP"/>. | FAAH plays a role in endocannabinoid signaling that has intriguing potential as a drug target. This signaling system consists of endocannabinoid ligands (such as AEA), two G protein-coupled receptors (CB1 and CB2), and the enzymes that synthesize and degrade (such as FAAH) the signaling lipids. Previous research has explored the potential of regulating endocannabinoid signaling through the CB1 and CB2 receptors. However, molecules found to activate these receptors (such as [http://www.ch.ic.ac.uk/vchemlib/mim/bristol/thc/thc_text.htm tetrahydrocannabinol] (THC), the main psychoactive ingredient of [http://en.wikipedia.org/wiki/Cannabis_(drug) marijuana]), while providing the intended pain relief, also produce many undesirable side effects, such as decreased cognition and motor control. On the other hand, research involving FAAH inhibitors has shown that blocking this part of the pathway reduces pain without the unwanted side effects seen through CB1/CB2 activation. Thus, exploring the possibility of using FAAH inhibition to decrease pain relief with minimal side effects could lead to new pain treatment solutions. For example, the [http://drugdiscoveryopinion.com/2009/04/pf-3845-%E2%80%93-a-potent-and-selective-faah-inhibitor/ PF-3845 inhibitor]of FAAH is extremely selective for the enzyme and raises AEA levels for a prolonged duration of time. Therefore, this inhibitor could be explored as a possible pharmaceutical product to target FAAH when extended pain relief is desired <ref name="2WAP"/>. | ||
| + | |||
| + | == 3D Structures of fatty acid amide hydrolase == | ||
| + | |||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | |||
| + | [[3qj8]] – rFAAH - rat<BR /> | ||
| + | [[1mt5]], [[3qj9]], [[3qkv]], [[4hbp]] – rFAAH + inhibitor <BR /> | ||
| + | [[2vya]], [[2wap]], [[2wj1]], [[2wj2]], [[3k7f]], [[3k83]], [[3k84]], [[3lj6]], [[3lj7]], [[3qk5]], [[3oj8]], [[3ppm]], [[3pr0]], [[4j5p]], [[6mrg]] – rFAAH (mutant) + inhibitor <BR /> | ||
| + | [[4do3]] – rFAAH + anti-inflammatory drug<BR /> | ||
| + | [[6dhv]] – AtFAAH – ''Arabidopsis thaliana''<BR /> | ||
| + | [[6dii]] –AtFAAH + methyl linolenyl fluorophosphonate<BR /> | ||
| + | |||
</StructureSection> | </StructureSection> | ||
| - | __NOTOC__ | ||
==References== | ==References== | ||
| Line 56: | Line 66: | ||
*Carter Sharp | *Carter Sharp | ||
| - | == 3D Structures of fatty acid amide hydrolase == | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | |||
| - | [[3qj8]] – rFAAH - rat<BR /> | ||
| - | [[1mt5]], [[3qj9]], [[3qkv]], [[4hbp]] – rFAAH + inhibitor <BR /> | ||
| - | [[2vya]], [[2wap]], [[2wj1]], [[2wj2]], [[3k7f]], [[3k83]], [[3k84]], [[3lj6]], [[3lj7]], [[3qk5]], [[3oj8]], [[3ppm]], [[3pr0]], [[4j5p]] – rFAAH (mutant) + inhibitor <BR /> | ||
| - | [[4do3]] – rFAAH + anti-inflammatory drug<BR /> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
[[Category:Featured in BAMBED]] | [[Category:Featured in BAMBED]] | ||
Revision as of 08:59, 26 June 2019
This page, as it appeared on May 3, 2014, was featured in this article in the journal Biochemistry and Molecular Biology Education.
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science. 2002 Nov 29;298(5599):1793-6. PMID:12459591 doi:10.1126/science.1076535
- ↑ 2.0 2.1 2.2 2.3 Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009 Apr 24;16(4):411-20. PMID:19389627 doi:10.1016/j.chembiol.2009.02.013
- ↑ 3.0 3.1 3.2 3.3 Mileni M, Johnson DS, Wang Z, Everdeen DS, Liimatta M, Pabst B, Bhattacharya K, Nugent RA, Kamtekar S, Cravatt BF, Ahn K, Stevens RC. Structure-guided inhibitor design for human FAAH by interspecies active site conversion. Proc Natl Acad Sci U S A. 2008 Sep 2;105(35):12820-4. Epub 2008 Aug 27. PMID:18753625
- ↑ Mileni M, Garfunkle J, DeMartino JK, Cravatt BF, Boger DL, Stevens RC. Binding and inactivation mechanism of a humanized fatty acid amide hydrolase by alpha-ketoheterocycle inhibitors revealed from cocrystal structures. J Am Chem Soc. 2009 Aug 5;131(30):10497-506. PMID:19722626 doi:10.1021/ja902694n
- ↑ 5.0 5.1 5.2 Mileni M, Kamtekar S, Wood DC, Benson TE, Cravatt BF, Stevens RC. Crystal structure of fatty acid amide hydrolase bound to the carbamate inhibitor URB597: discovery of a deacylating water molecule and insight into enzyme inactivation. J Mol Biol. 2010 Jul 23;400(4):743-54. Epub 2010 May 21. PMID:20493882 doi:10.1016/j.jmb.2010.05.034
Similar Proteopedia Pages
Student Contributors
- Rachel Erkilla
- Melissa Jones
- Daniel Lange
- Carter Sharp
Proteopedia Page Contributors and Editors (what is this?)
R. Jeremy Johnson, Michal Harel, Alexander Berchansky, Angel Herraez