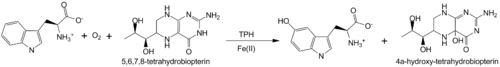We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Hydroxylase
From Proteopedia
(Difference between revisions)
| Line 41: | Line 41: | ||
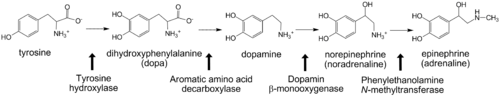
Deficiency in tyrosine hydroxylase has been observed in dopa responsive dystonia and juvenile parkinsonism. A reduced 3,4-dihydroxyphenylalanine production is also observed in Parkinson’s disease<ref>PMID: 11849022</ref>. | Deficiency in tyrosine hydroxylase has been observed in dopa responsive dystonia and juvenile parkinsonism. A reduced 3,4-dihydroxyphenylalanine production is also observed in Parkinson’s disease<ref>PMID: 11849022</ref>. | ||
| + | |||
| + | ==3D structures of hydroxylase== | ||
| + | [[Hydroxylases 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
==3D structures of hydroxylases== | ==3D structures of hydroxylases== | ||
| Line 48: | Line 52: | ||
*Phenylalanine hydroxylase | *Phenylalanine hydroxylase | ||
| - | **[[ | + | **[[6n1k]] – hPAH (mutant) – human<br /> |
| + | **[[6hyc]] – hPAH + cofactor<br /> | ||
**[[1j8t]], [[1pah]], [[2pah]] - hPAH catalytic domain<br /> | **[[1j8t]], [[1pah]], [[2pah]] - hPAH catalytic domain<br /> | ||
| + | **[[1tdw]] – hPAH catalytic domain (mutant) <br /> | ||
| + | **[[6hpo]] – hPAH catalytic domain + Fe <br /> | ||
**[[3pah]], [[4pah]] – hPAH catalytic domain + adrenaline inhibitor<br /> | **[[3pah]], [[4pah]] – hPAH catalytic domain + adrenaline inhibitor<br /> | ||
**[[5pah]], [[6pah]] - hPAH catalytic domain + dopamine inhibitor<br /> | **[[5pah]], [[6pah]] - hPAH catalytic domain + dopamine inhibitor<br /> | ||
**[[1j8u]] - hPAH catalytic domain + cofactor<br /> | **[[1j8u]] - hPAH catalytic domain + cofactor<br /> | ||
| - | **[[1dmw]] - hPAH + cofactor<br /> | + | **[[1dmw]] - hPAH catalytic domain + cofactor<br /> |
| - | **[[1tg2]], [[1lrm]] - hPAH (mutant) + cofactor<br /> | + | **[[6hpo]] - hPAH catalytic domain + cofactor + Fe<br /> |
| + | **[[1tg2]], [[1lrm]] - hPAH catalytic domain (mutant) + cofactor<br /> | ||
**[[1mmk]], [[1mmt]], [[1kw0]] - hPAH catalytic domain + cofactor + substrate<br /> | **[[1mmk]], [[1mmt]], [[1kw0]] - hPAH catalytic domain + cofactor + substrate<br /> | ||
**[[4anp]] - hPAH catalytic domain + pyrimidine derivative<br /> | **[[4anp]] - hPAH catalytic domain + pyrimidine derivative<br /> | ||
**[[5fii]], [[5fgi]] - hPAH residues 19-118 + phenylalanine<br /> | **[[5fii]], [[5fgi]] - hPAH residues 19-118 + phenylalanine<br /> | ||
| + | **[[1phz]], [[2phm]], [[5den]], [[5fgj]], [[5den]] – rPAH - rat<br /> | ||
| + | **[[5egq]] - rPAH (mutant) <br /> | ||
**[[5jk6]] - smPAH + Fe – slime mold<br /> | **[[5jk6]] - smPAH + Fe – slime mold<br /> | ||
**[[5jk5]] - smPAH + cofactor + Fe <br /> | **[[5jk5]] - smPAH + cofactor + Fe <br /> | ||
| Line 105: | Line 115: | ||
**[[1k0l]] – PaPHBH (mutant) + FAD <br /> | **[[1k0l]] – PaPHBH (mutant) + FAD <br /> | ||
**[[6dll]] – PfPHBH + FAD – ''Pseudomonas putida''<br /> | **[[6dll]] – PfPHBH + FAD – ''Pseudomonas putida''<br /> | ||
| + | **[[2dki]] – CtPHBH + FAD – ''Comamonas testosteroni''<br /> | ||
| + | **[[2dkh]] – CtPHBH + FAD + hydroxy-benzoate <br /> | ||
*Apartyl/asparaginyl β-hydroxylase | *Apartyl/asparaginyl β-hydroxylase | ||
| Line 116: | Line 128: | ||
*Alpha-ketoglutarate-dependent L-arginine hydroxylase | *Alpha-ketoglutarate-dependent L-arginine hydroxylase | ||
| - | **[[6dax]], [[6alm]], [[6aln]] – SvAAH + arginine + oxoglutarate + Fe – ''Streptomyces vinaceus''<br /> | + | **[[6dax]], [[6alm]], [[6aln]], [[6mp8]] – SvAAH + arginine + oxoglutarate + Fe – ''Streptomyces vinaceus''<br /> |
**[[6daz]], [[6alo]], [[6alp]], [[6alq]] – SvAAH + arginine + succinate + Fe <br /> | **[[6daz]], [[6alo]], [[6alp]], [[6alq]] – SvAAH + arginine + succinate + Fe <br /> | ||
**[[6db2]], [[6alr]] – SvAAH + homoarginine + succinate + V <br /> | **[[6db2]], [[6alr]] – SvAAH + homoarginine + succinate + V <br /> | ||
| + | **[[6mp9]] – SvAAH + guanidinopentanoate + succinate + Fe <br /> | ||
*PLP-dependent L-arginine hydroxylase | *PLP-dependent L-arginine hydroxylase | ||
| Line 125: | Line 138: | ||
**[[5dj1]] – SwAAH + PLP + Mg<br /> | **[[5dj1]] – SwAAH + PLP + Mg<br /> | ||
**[[5dj3]] – SwAAH + PLP-arginine + Mg<br /> | **[[5dj3]] – SwAAH + PLP-arginine + Mg<br /> | ||
| + | **[[5bk7]] – SwAAH (mutant) + PLP-arginine <br /> | ||
| + | **[[6c8t]], [[6c92]] – SwAAH + PLP-arginine + PLP derivative<br /> | ||
*Leucine hydroxylase | *Leucine hydroxylase | ||
| Line 131: | Line 146: | ||
**[[5nci]] – StAAH + leucine derivative + oxoglutarate + Co <br /> | **[[5nci]] – StAAH + leucine derivative + oxoglutarate + Co <br /> | ||
**[[5ncj]] – StAAH + leucine derivative + succinate + Mn <br /> | **[[5ncj]] – StAAH + leucine derivative + succinate + Mn <br /> | ||
| + | |||
| + | *L-arginine b-hydroxylase or non-heme iron oxygenase | ||
| + | |||
| + | **[[4m23]] – SlORFP – ''Streptomyces lavendulae''<br /> | ||
| + | **[[4m2i]] – SlORFP + Fe <br /> | ||
| + | **[[4m25]] – SlORFP + Fe + ketoglutarate <br /> | ||
| + | **[[4m27]], [[4m2c]] – SlORFP + Fe + Arg<br /> | ||
| + | **[[4m2e]] – SlORFP + Fe + Arg derivative<br /> | ||
| + | **[[4m2f]] – SlORFP + Fe + canavanine<br /> | ||
| + | **[[4m2g]], [[4m26]] – SlORFP + Fe + succinate + Arg derivative<br /> | ||
| + | **[[2wbo]] – SvVIOC + Fe + Arg – ''Streptomyces vinaceus''<br /> | ||
| + | **[[2wbq]] – SvVIOC + Arg derivative<br /> | ||
| + | **[[2wbp]] – SvVIOC + Fe + succinate + Arg derivative<br /> | ||
*Prolyl 4-hydroxylase | *Prolyl 4-hydroxylase | ||
| + | **[[4bt8]], [[2yq8]] - hPH a1 subunit collagen-binding domain<br /> | ||
| + | **[[1tjc]] - hPH a1 subunit collagen-binding domain (mutant)<br /> | ||
| + | **[[4btb]], [[4bta]], [[4bt9]] - hPH a1 subunit + peptide<br /> | ||
| + | **[[6evl]] - hPH a2 subunit <br /> | ||
| + | **[[6evm]], [[6evn]], [[6evo]], [[6evp]] - hPH a2 subunit + peptide<br /> | ||
**[[5hv0]] - PH + Cd – ''Bacillus anthracis'' <br /> | **[[5hv0]] - PH + Cd – ''Bacillus anthracis'' <br /> | ||
**[[5c5u]] - PcvPH + Mn – Paramecium chlorella virus <br /> | **[[5c5u]] - PcvPH + Mn – Paramecium chlorella virus <br /> | ||
| Line 139: | Line 172: | ||
**[[4p7x]] - RlPH + Co + pipecolic acid – ''Rhizobium loti'' <br /> | **[[4p7x]] - RlPH + Co + pipecolic acid – ''Rhizobium loti'' <br /> | ||
**[[4p7w]] - RlPH + Co + proline<br /> | **[[4p7w]] - RlPH + Co + proline<br /> | ||
| + | **[[6iuq]] - PH + Fe – ''Phytophthora capsici''<br /> | ||
| + | **[[6ey1]] - TaPH + Mn – ''Trichoplax adhaerens''<br /> | ||
| + | **[[6f0w]] - TaPH + Mn + hypoxia inducible factor + oxalylglycine <br /> | ||
| + | **[[2v4a]], [[2jij]] - CrPH – ''Chlamydomonas reinhardtii'' <br /> | ||
| + | **[[2jig]] - CrPH + Zn + pyridine derivative<br /> | ||
| + | |||
| + | *HIF prolyl hydroxylase 1 or egl nine homolog 2 | ||
| + | |||
| + | **[[5v1b]] – hHPH-1 + Fe + inhibitor <br /> | ||
| + | |||
| + | *HIF prolyl hydroxylase 2 or egl nine homolog 1 | ||
| + | |||
| + | **[[5las]] – hHPH-2 + HIF <br /> | ||
| + | **[[5l9b]] – hHPH-2 catalytic domain (mutant) + Mn + HIF + oxoglutarate <br /> | ||
| + | **[[5l9v]], [[5la9]], [[3hqr]], [[3hqu]] – hHPH-2 catalytic domain (mutant) + Mn + HIF + oxalylglycine <br /> | ||
| + | **[[5ox5]], [[5ox6]], [[4bqw]], [[4bqx]], [[4bqy]] – hHPH-2 catalytic domain + Mn + inhibitor <br /> | ||
| + | **[[5l9r]] – hHPH-2 catalytic domain + Mn + oxalylglycine <br /> | ||
| + | **[[5v18]], [[6nmq]], [[3ouh]], [[3oui]], [[3ouj]], [[2y34]], [[2hbt]], [[2hbu]], [[2g19]], [[2g1m]] – hHPH-2 catalytic domain + Fe + inhibitor <br /> | ||
| + | **[[4jzr]] – hHPH-2 catalytic domain + Ni + inhibitor <br /> | ||
| + | **[[2y33]] – hHPH-2 catalytic domain + Zn + inhibitor <br /> | ||
| + | **[[5lat]], [[5lb6]], [[5lbb]], [[5lbc]], [[5lbe]], [[5lbf]], [[5a3u]], [[4uwd]], [[4kbz]] – hHPH-2 catalytic domain (mutant) + Mn + inhibitor <br /> | ||
| + | |||
| + | *Lysine 4-hydroxylase | ||
| + | |||
| + | **[[6f9p]] – FlL4H + Fe – ''Flavobacterium'' <br /> | ||
| + | **[[6exf]] – FlL4H + Fe + lysine <br /> | ||
| + | **[[6euo]] – FlL4H + Fe + succinate derivative<br /> | ||
| + | **[[6eur]] – FlL4H + Fe + oxoglutarate<br /> | ||
| + | **[[6exh]] – FlL4H + Fe + lysine + succinate<br /> | ||
| + | |||
| + | *Lysine 3-hydroxylase | ||
| + | |||
| + | **[[6f2e]] – CaL3H – ''Catenulispora acidiphila'' <br /> | ||
| + | **[[6f2b]] – CaL3H + Fe + oxoglutarate <br /> | ||
| + | **[[6f2a]] – CaL3H + Fe + lysine <br /> | ||
| + | **[[6f6j]] – CaL3H + Fe + succinate + hydroxyl lysine <br /> | ||
*Salicilate hydroxylase | *Salicilate hydroxylase | ||
Revision as of 09:43, 25 August 2019
| |||||||||||
3D structures of hydroxylases
Updated on 25-August-2019
Additional Resources
For additional information, see: Amino Acid Synthesis & Metabolism
References
- ↑ Fitzpatrick PF. The aromatic amino acid hydroxylases. Adv Enzymol Relat Areas Mol Biol. 2000;74:235-94. PMID:10800597
- ↑ KAUFMAN S. The enzymatic conversion of phenylalanine to tyrosine. J Biol Chem. 1957 May;226(1):511-24. PMID:13428782
- ↑ Chen D, Frey PA. Phenylalanine hydroxylase from Chromobacterium violaceum. Uncoupled oxidation of tetrahydropterin and the role of iron in hyroxylation. J Biol Chem. 1998 Oct 2;273(40):25594-601. PMID:9748224
- ↑ Leiros HK, Pey AL, Innselset M, Moe E, Leiros I, Steen IH, Martinez A. Structure of phenylalanine hydroxylase from Colwellia psychrerythraea 34H, a monomeric cold active enzyme with local flexibility around the active site and high overall stability. J Biol Chem. 2007 Jul 27;282(30):21973-86. Epub 2007 May 30. PMID:17537732 doi:10.1074/jbc.M610174200
- ↑ Grenett HE, Ledley FD, Reed LL, Woo SL. Full-length cDNA for rabbit tryptophan hydroxylase: functional domains and evolution of aromatic amino acid hydroxylases. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5530-4. PMID:3475690
- ↑ NAGATSU T, LEVITT M, UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910-7. PMID:14216443
- ↑ 7.0 7.1 LEVINE RJ, LOVENBERG W, SJOERDSMA A. HYDROXYLATION OF TRYPTOPHAN AND PHENYLALANINE IN NEOPLASTIC MAST CELLS OF THE MOUSE. Biochem Pharmacol. 1964 Sep;13:1283-90. PMID:14221726
- ↑ Grahame-Smith DG. Tryptophan hydroxylation in brain. Biochem Biophys Res Commun. 1964 Aug 11;16(6):586-92. PMID:5297063
- ↑ 9.0 9.1 Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003 Nov 1;66(9):1673-80. PMID:14563478
- ↑ Grenett HE, Ledley FD, Reed LL, Woo SL. Full-length cDNA for rabbit tryptophan hydroxylase: functional domains and evolution of aromatic amino acid hydroxylases. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5530-4. PMID:3475690
- ↑ Craig SP, Boularand S, Darmon MC, Mallet J, Craig IW. Localization of human tryptophan hydroxylase (TPH) to chromosome 11p15.3----p14 by in situ hybridization. Cytogenet Cell Genet. 1991;56(3-4):157-9. PMID:2055111
- ↑ Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003 Jan 3;299(5603):76. PMID:12511643 doi:http://dx.doi.org/10.1126/science.1078197
- ↑ 13.0 13.1 Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry. 2004 Feb 15;55(4):428-33. PMID:14960297 doi:http://dx.doi.org/10.1016/j.biopsych.2003.09.002
- ↑ Slominski A, Pisarchik A, Johansson O, Jing C, Semak I, Slugocki G, Wortsman J. Tryptophan hydroxylase expression in human skin cells. Biochim Biophys Acta. 2003 Oct 15;1639(2):80-6. PMID:14559114
- ↑ Hasegawa H, Yanagisawa M, Inoue F, Yanaihara N, Ichiyama A. Demonstration of non-neural tryptophan 5-mono-oxygenase in mouse intestinal mucosa. Biochem J. 1987 Dec 1;248(2):501-9. PMID:3435461
- ↑ Hosoda S, Nakamura W, Takatsuki K. Properties of tryptophan hydroxylase from human carcinoid tumor. Biochim Biophys Acta. 1977 May 12;482(1):27-34. PMID:16654
- ↑ Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry. 1999 Mar 1;45(5):603-14. PMID:10088047
- ↑ Mitchell JJ, Trakadis YJ, Scriver CR. Phenylalanine hydroxylase deficiency. Genet Med. 2011 Aug;13(8):697-707. doi: 10.1097/GIM.0b013e3182141b48. PMID:21555948 doi:http://dx.doi.org/10.1097/GIM.0b013e3182141b48
- ↑ Flatmark T, Stevens RC. Structural Insight into the Aromatic Amino Acid Hydroxylases and Their Disease-Related Mutant Forms. Chem Rev. 1999 Aug 11;99(8):2137-2160. PMID:11849022
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Michael Skovbo Windahl, David Canner, Joel L. Sussman, Jaime Prilusky