We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Transferrin
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== Structural highlights == | == Structural highlights == | ||
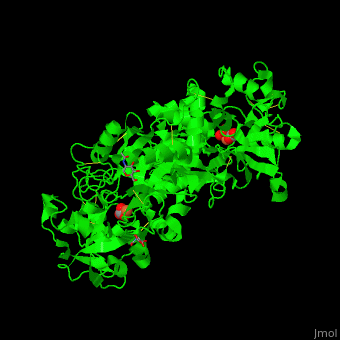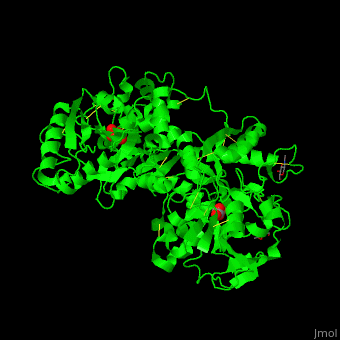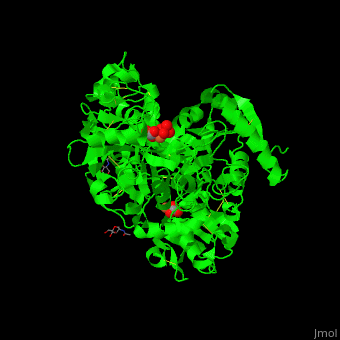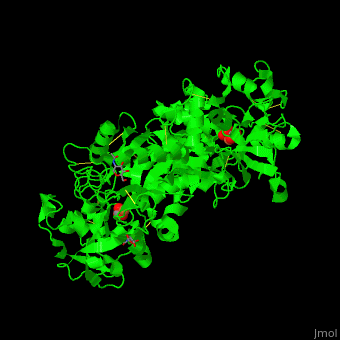
| - | TF contains <scene name='48/480879/Cv/ | + | TF contains <scene name='48/480879/Cv/7'>two lobes</scene>: at the N and C termini. The Fe+3 ion is coordinated to several side chains belonging to both N- and C-lobe, carbonate and sulfate ions<ref>PMID:23256035</ref>. |
| - | *<scene name='48/480879/Cv/ | + | *<scene name='48/480879/Cv/8'>N-lobe Fe coordination site</scene>. |
| - | *<scene name='48/480879/Cv/ | + | *<scene name='48/480879/Cv/9'>C-lobe Fe coordination site</scene>. |
</StructureSection> | </StructureSection> | ||
== 3D Structures of Transferrin == | == 3D Structures of Transferrin == | ||
Revision as of 12:40, 25 September 2019
| |||||||||||
3D Structures of Transferrin
Updated on 25-September-2019
References
- ↑ de Jong G, van Dijk JP, van Eijk HG. The biology of transferrin. Clin Chim Acta. 1990 Sep;190(1-2):1-46. PMID:2208733
- ↑ Liu YS, Xu GY, Cheng DQ, Li YM. Determination of serum carbohydrate-deficient transferrin in the diagnosis of alcoholic liver disease. Hepatobiliary Pancreat Dis Int. 2005 May;4(2):265-8. PMID:15908327
- ↑ Yang N, Zhang H, Wang M, Hao Q, Sun H. Iron and bismuth bound human serum transferrin reveals a partially-opened conformation in the N-lobe. Sci Rep. 2012;2:999. doi: 10.1038/srep00999. Epub 2012 Dec 19. PMID:23256035 doi:10.1038/srep00999