Trypanothione reductase
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structural highlights == | == Structural highlights == | ||
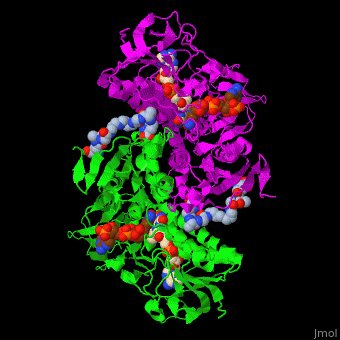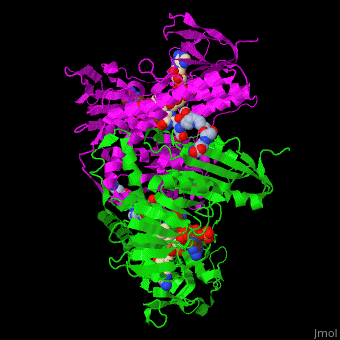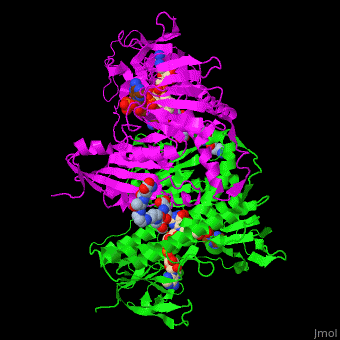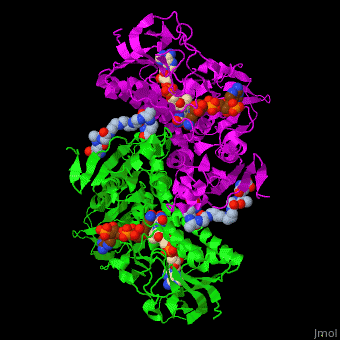
| - | The <scene name='48/489332/Cv/ | + | The <scene name='48/489332/Cv/6'>active site of TTR</scene> contains <scene name='48/489332/Cv/7'>two catalytic cysteine</scene> residues which are part of the electron transfer function of the enzyme and the bound TPT substrate<ref>PMID:23733388</ref>. |
</StructureSection> | </StructureSection> | ||
Revision as of 13:06, 2 October 2019
| |||||||||||
3D structures of trypanothione reductase
Updated on 02-October-2019
References
- ↑ Walsh C, Bradley M, Nadeau K. Molecular studies on trypanothione reductase, a target for antiparasitic drugs. Trends Biochem Sci. 1991 Aug;16(8):305-9. PMID:1957352
- ↑ Rivarola HW, Paglini-Oliva PA. Trypanosoma cruzi trypanothione reductase inhibitors: phenothiazines and related compounds modify experimental Chagas' disease evolution. Curr Drug Targets Cardiovasc Haematol Disord. 2002 Jun;2(1):43-52. PMID:12769656
- ↑ Baiocco P, Poce G, Alfonso S, Cocozza M, Porretta GC, Colotti G, Biava M, Moraca F, Botta M, Yardley V, Fiorillo A, Lantella A, Malatesta F, Ilari A. Inhibition of Leishmania infantum Trypanothione Reductase by Azole-Based Compounds: a Comparative Analysis with Its Physiological Substrate by X-ray Crystallography. ChemMedChem. 2013 Jun 3. doi: 10.1002/cmdc.201300176. PMID:23733388 doi:10.1002/cmdc.201300176