Iron sulfur proteins
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
<scene name='49/492892/Cv/11'>Fe4S4 center and 2 Ni+2 ions form interactions with 6 cysteine residues</scene> in Acetyl-CoA synthase IV subunit α from ''Carboxydothermus hydrogenoformans'' ([[1ru3]]).<ref>PMID:14699043</ref> Water molecules shown as red spheres. | <scene name='49/492892/Cv/11'>Fe4S4 center and 2 Ni+2 ions form interactions with 6 cysteine residues</scene> in Acetyl-CoA synthase IV subunit α from ''Carboxydothermus hydrogenoformans'' ([[1ru3]]).<ref>PMID:14699043</ref> Water molecules shown as red spheres. | ||
| - | === {{nowrap|N-Butylisocyanide Oxidation}} at the {{nowrap|[NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>]-cluster}} of CO Dehydrogenase | + | === {{nowrap|N-Butylisocyanide Oxidation}} at the {{nowrap|[NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>]-cluster}} of CO Dehydrogenase<ref>DOI 10.1007/s00775-011-0839-y</ref> === |
| - | + | ||
| - | + | ||
| - | + | ||
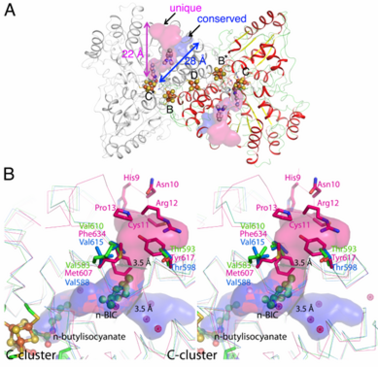
Ni, Fe-containing carbon monoxide dehydrogenases (CODHs) play an important role in anaerobic bacteria and archea by allowing them to grow with CO or CO<sub>2</sub> as their sole carbon and/or energy source. The <scene name='Journal:JBIC:13/Cv/6'>structures of CODHs are homodimers</scene> with ~ 130 kDa containing <scene name='Journal:JBIC:13/Cv/18'>five metal clusters</scene>, called B, B’, C, C’ and D. Each subunit contains the <scene name='Journal:JBIC:13/Cv/19'>active site C-cluster</scene> and cubane-type [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/20'>B-cluster</scene>. Another [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/22'>D-cluster</scene> connects two subunits forming a covalent homodimer. The CODHs catalyze the reversible oxidation of CO to CO2 at the active site C-cluster, which is composed of [NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>] (CO + H<sub>2</sub>O ↔ CO<sub>2</sub> + 2e– + 2H+). In addition to the reversible oxidation of CO, CODHs are able to catalyze further reactions, such as the oxidation of H<sub>2</sub> and the reductions of protons, 2,4,6-trinitrotoluene (TNT), and hydroxylamine, as well as the oxidation of n-butylisocyanide (n-BIC). N-BIC is a slow-turnover substrate of CODHs, whose oxidation occurs at the C-cluster. | Ni, Fe-containing carbon monoxide dehydrogenases (CODHs) play an important role in anaerobic bacteria and archea by allowing them to grow with CO or CO<sub>2</sub> as their sole carbon and/or energy source. The <scene name='Journal:JBIC:13/Cv/6'>structures of CODHs are homodimers</scene> with ~ 130 kDa containing <scene name='Journal:JBIC:13/Cv/18'>five metal clusters</scene>, called B, B’, C, C’ and D. Each subunit contains the <scene name='Journal:JBIC:13/Cv/19'>active site C-cluster</scene> and cubane-type [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/20'>B-cluster</scene>. Another [Fe<sub>4</sub>S<sub>4</sub>] <scene name='Journal:JBIC:13/Cv/22'>D-cluster</scene> connects two subunits forming a covalent homodimer. The CODHs catalyze the reversible oxidation of CO to CO2 at the active site C-cluster, which is composed of [NiFe<sub>4</sub>S<sub>4</sub>OH<sub>x</sub>] (CO + H<sub>2</sub>O ↔ CO<sub>2</sub> + 2e– + 2H+). In addition to the reversible oxidation of CO, CODHs are able to catalyze further reactions, such as the oxidation of H<sub>2</sub> and the reductions of protons, 2,4,6-trinitrotoluene (TNT), and hydroxylamine, as well as the oxidation of n-butylisocyanide (n-BIC). N-BIC is a slow-turnover substrate of CODHs, whose oxidation occurs at the C-cluster. | ||
Revision as of 13:36, 29 October 2019
| |||||||||||
References
- ↑ Iwasaki T, Kappl R, Bracic G, Shimizu N, Ohmori D, Kumasaka T. ISC-like [2Fe-2S] ferredoxin (FdxB) dimer from Pseudomonas putida JCM 20004: structural and electron-nuclear double resonance characterization. J Biol Inorg Chem. 2011 Jun 7. PMID:21647778 doi:10.1007/s00775-011-0793-8
- ↑ Rekittke I, Wiesner J, Rohrich R, Demmer U, Warkentin E, Xu W, Troschke K, Hintz M, No JH, Duin EC, Oldfield E, Jomaa H, Ermler U. Structure of (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate reductase, the terminal enzyme of the non-mevalonate pathway. J Am Chem Soc. 2008 Dec 24;130(51):17206-7. PMID:19035630 doi:http://dx.doi.org/10.1021/ja806668q
- ↑ Span I, Grawert T, Bacher A, Eisenreich W, Groll M. Crystal Structures of Mutant IspH Proteins Reveal a Rotation of the Substrate's Hydroxymethyl Group during Catalysis. J Mol Biol. 2011 Nov 23. PMID:22137895 doi:10.1016/j.jmb.2011.11.033
- ↑ Svetlitchnyi V, Dobbek H, Meyer-Klaucke W, Meins T, Thiele B, Romer P, Huber R, Meyer O. A functional Ni-Ni-[4Fe-4S] cluster in the monomeric acetyl-CoA synthase from Carboxydothermus hydrogenoformans. Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):446-51. Epub 2003 Dec 29. PMID:14699043 doi:10.1073/pnas.0304262101
- ↑ Jeoung JH, Dobbek H. n-Butyl isocyanide oxidation at the [NiFe(4)S (4)OH ( x )] cluster of CO dehydrogenase. J Biol Inorg Chem. 2011 Sep 9. PMID:21904889 doi:10.1007/s00775-011-0839-y
- ↑ Lovgreen MN, Martic M, Windahl MS, Christensen HE, Harris P. Crystal structures of the all-cysteinyl-coordinated D14C variant of Pyrococcus furiosus ferredoxin: [4Fe-4S] <--> [3Fe-4S] cluster conversion. J Biol Inorg Chem. 2011 Apr 12. PMID:21484348 doi:10.1007/s00775-011-0778-7
![Skewed orientations of the gmax component (red) with respect to the molecular frame of the [2Fe–2S] cluster of FdxB.](/wiki/images/2/29/FdxBFig8.jpg)
![pH dependent equilibrium of D14C [3Fe-4S] P. furiosus ferredoxin between protonated and deprotonated monomers and formation of a disulfide bonded dimer from deprotonated monomers. Fd is short for ferredoxin.](/wiki/images/thumb/0/0b/Schem1.png/300px-Schem1.png)
