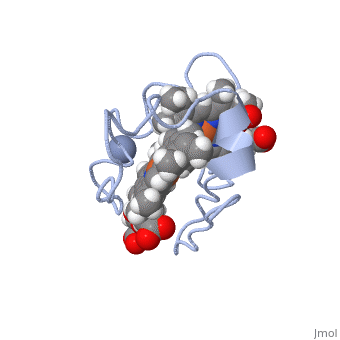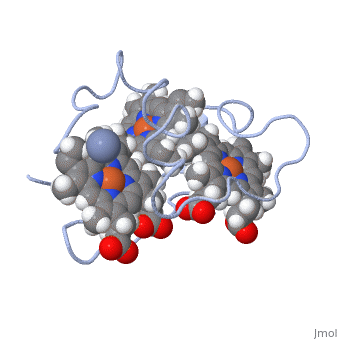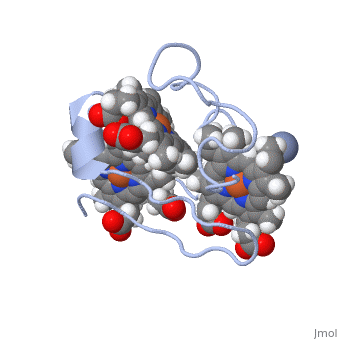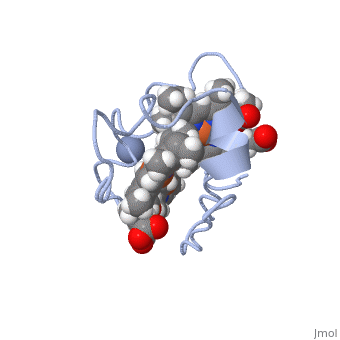We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Cytochrome c 7
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
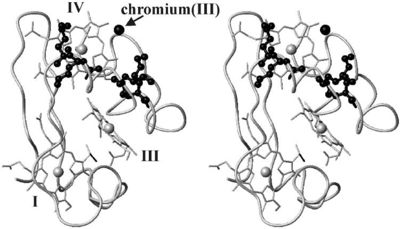
To begin the reaction, Cc7 must be in its resting state (i.e. fully reduced). A negatively charged chromate (-2) interacts and binds with the positively charged surface area of Cc7 containing the positive lysine residues located on the distal side of heme IV. Lysine residues 41, 42, 46 and 50 form the binding site for the chromate (-2) ion. The first round of reductions takes place once the chromate (-2) ion binds to the active site. Binding of the chromate (-2) ion induces the iron (II) atom of heme groups I and III to be oxidized first. Because of the close proximity of all the heme groups, the oxidation of heme groups I and III causes heme group IV to reduce the chromate (-2) ion. | To begin the reaction, Cc7 must be in its resting state (i.e. fully reduced). A negatively charged chromate (-2) interacts and binds with the positively charged surface area of Cc7 containing the positive lysine residues located on the distal side of heme IV. Lysine residues 41, 42, 46 and 50 form the binding site for the chromate (-2) ion. The first round of reductions takes place once the chromate (-2) ion binds to the active site. Binding of the chromate (-2) ion induces the iron (II) atom of heme groups I and III to be oxidized first. Because of the close proximity of all the heme groups, the oxidation of heme groups I and III causes heme group IV to reduce the chromate (-2) ion. | ||
| - | [[Image:Tileshop.fcgi.jpg| | + | [[Image:Tileshop.fcgi.jpg|400px|alt=Alt text|Figure 1<ref name="assfalt" />: The binding of chromate (-2) to the active site causes a conformational change in Cc7, resulting in the repositioning of heme groups I, III, and IV which induces reductase enzymatic activity.]] |
To put it simply, the heme groups I and III push the electron from heme group IV into the chromate (-2) ion. The process continues sequentially from heme III to heme I as each electron from their iron (II) are donated to chromium atom via heme IV. This ability is called '''electron tunnelling'''<ref name="assfalt" /> and it believed to be the main driving force for this reaction to occur. This is thought to be possible as heme groups I and III are able to re-establish thermodynamic conditions by transferring their free iron (II) electron to heme IV. The final result of this series of reductions is a chromium (III) ion. The chromium (III) ion remains in the same region where the charged chromate (-2) ion was first bound. This is made possible by the positive lysine residues present in the active site. The positive nature of the lysine side chains binds the cation in place. | To put it simply, the heme groups I and III push the electron from heme group IV into the chromate (-2) ion. The process continues sequentially from heme III to heme I as each electron from their iron (II) are donated to chromium atom via heme IV. This ability is called '''electron tunnelling'''<ref name="assfalt" /> and it believed to be the main driving force for this reaction to occur. This is thought to be possible as heme groups I and III are able to re-establish thermodynamic conditions by transferring their free iron (II) electron to heme IV. The final result of this series of reductions is a chromium (III) ion. The chromium (III) ion remains in the same region where the charged chromate (-2) ion was first bound. This is made possible by the positive lysine residues present in the active site. The positive nature of the lysine side chains binds the cation in place. | ||
Current revision
General
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Assfalg M, Bertini I, Bruschi M, Michel C, Turano P. The metal reductase activity of some multiheme cytochromes c: NMR structural characterization of the reduction of chromium(VI) to chromium(III) by cytochrome c(7). 2002; 99(15):9750-4 DOI: 10.1073/pnas.152290999
- ↑ Barton L, Fauque G. Biochemistry, Physiology and Biotechnology of Sulfate-Reducing Bacteria. Advances in Applied Microbiology. 2009; 68: 41–98. DOI: 10.1016/s0065-2164(09)01202-7
- ↑ 3.0 3.1 Pfennig N, Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. 1976; 110(1): 3-12 DOI: 10.1007/BF00416962