We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Nitrogenase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
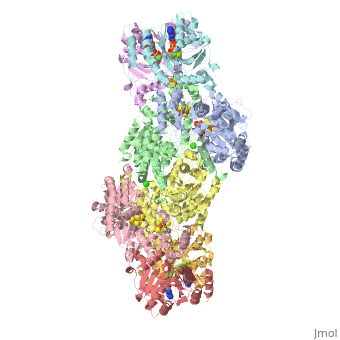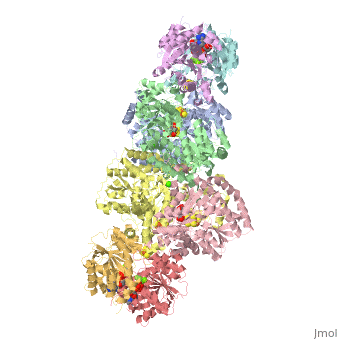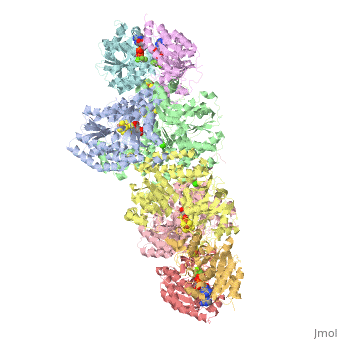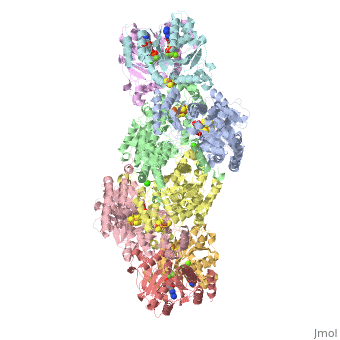
<StructureSection load="1N2C" size="350" color="white" caption="Nitrogenase complex: α (grey and green) and β (pink and yellow) chains, nitrogenase iron protein ( purple,cyan, red and gold), showing Fe-Mo-S cluster complex with ADP, adipic acid, AlF4, Ca+2 and Mg+2 ions [[1n2c]]"> | <StructureSection load="1N2C" size="350" color="white" caption="Nitrogenase complex: α (grey and green) and β (pink and yellow) chains, nitrogenase iron protein ( purple,cyan, red and gold), showing Fe-Mo-S cluster complex with ADP, adipic acid, AlF4, Ca+2 and Mg+2 ions [[1n2c]]"> | ||
| + | __TOC__ | ||
| + | ==Function== | ||
| + | |||
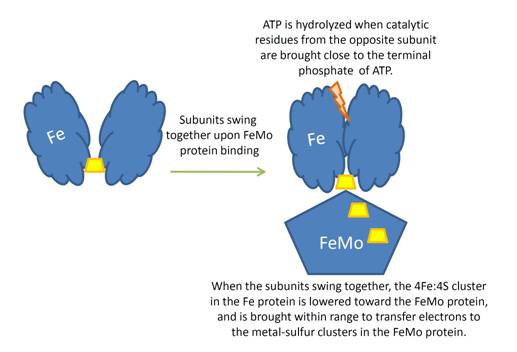
'''Nitrogenase''' (Nase) is an enzyme that fixes atmospheric nitrogen (N<sub>2</sub>) into ammonia. Though abundantly present in the atmosphere, most organisms cannot utilize N<sub>2</sub> directly, and must instead take it in through other forms, like ammonia or nitrate. The triple bond in N<sub>2</sub> is highly resistant to changes in oxidation state, and nitrogenases, found only in nitrogen-fixing bacteria, are the only proteins capable of reducing N<sub>2</sub> to ammonia. | '''Nitrogenase''' (Nase) is an enzyme that fixes atmospheric nitrogen (N<sub>2</sub>) into ammonia. Though abundantly present in the atmosphere, most organisms cannot utilize N<sub>2</sub> directly, and must instead take it in through other forms, like ammonia or nitrate. The triple bond in N<sub>2</sub> is highly resistant to changes in oxidation state, and nitrogenases, found only in nitrogen-fixing bacteria, are the only proteins capable of reducing N<sub>2</sub> to ammonia. | ||
| Line 21: | Line 24: | ||
For these reasons, binding of Fe protein to FeMo protein results in hydrolysis of ATP. Additionally, the 4Fe:4S cluster is lowered close enough to the metal-sulfur clusters of the FeMo protein that electron transfer can occur. All three clusters found in the Fe protein-FeMo protein complex can be seen <scene name='Sandbox_10/1n2c_clusters/2'>here</scene>. Once the electrons have passed from the 4Fe:4S cluster of the Fe protein to the 8Fe:7S cluster of the FeMo protein, they then transfer to the 7Fe:Mo:9S:homocitrate:X cluster where X is an unidentified light atom. It is at this cluster where reduction of N<sub>2</sub> and H<sup>+</sup> occur. The exact mechanism of reduction, however, is still unknown. | For these reasons, binding of Fe protein to FeMo protein results in hydrolysis of ATP. Additionally, the 4Fe:4S cluster is lowered close enough to the metal-sulfur clusters of the FeMo protein that electron transfer can occur. All three clusters found in the Fe protein-FeMo protein complex can be seen <scene name='Sandbox_10/1n2c_clusters/2'>here</scene>. Once the electrons have passed from the 4Fe:4S cluster of the Fe protein to the 8Fe:7S cluster of the FeMo protein, they then transfer to the 7Fe:Mo:9S:homocitrate:X cluster where X is an unidentified light atom. It is at this cluster where reduction of N<sub>2</sub> and H<sup>+</sup> occur. The exact mechanism of reduction, however, is still unknown. | ||
| + | |||
==3D structure of Nitrogenase== | ==3D structure of Nitrogenase== | ||
[[Nitrogenase 3D structures]] | [[Nitrogenase 3D structures]] | ||
Revision as of 08:45, 11 November 2019
| |||||||||||
3D structure of Nitrogenase
Updated on 11-November-2019 {{#tree:id=OrganizedByTopic|openlevels=0|
- Nase Mo-Fe
- 3pdi – AvNase α+β subunit [Fe4S4 Fe8S9] – Azotobacter vinelandii
- 2kic – AvNase γ subunit – NMR
- 3k1a, 1m1n, 2min, 3min, 3u7q – AvNase α+β subunit [CFe7MoS9 Fe8S7]
- 4xpi, 5cx1 - AvNase + (mutant) subunit [CFe7MoS9 Fe8S7]
- 4tku, 5bvg - AvNase α+β subunit [CFe7MoS9 Fe8S7 Fe2]
- 6o7o, 6o7m, 6o7l, 6o7p, 6o7q, 6o7r - AvNase + (mutant) subunit [CFe7MoS9 Fe8S7 Fe2]
- 6o7s - AvNase + (mutant) subunit [CFe7MoS9 Fe6S7 Fe2]
- 6op4 - AvNase + subunit [CFe7MoS9 Fe8S7 Mg]
- 4tkv, 5bvh- AvNase α+β subunit [CFe7MoS9 Fe8S7 Fe2] + CO
- 6op1 - AvNase + subunit [CFe7MoS9 Fe8S7 Mg] + CO
- 6op2, 60p3 - AvNase + subunit [CFe7MoS9 Fe8S7 Se] +
- 4wza - AvNase α+β subunit [CFe7MoS9 Fe8S7 Fe4S4 Fe2] + ADP + ACP
- 4wzb - AvNase α+β subunit [CFe7MoS9 Fe8S7 Fe4S4 Fe2] + ACP
- 4nd8, 6bbl, 6cdk - AvNase α+β subunit [CFe7MoS9 Fe8S7 Fe+3]
- 1l5h - AvNase α+β subunit [Fe8S7]
- 1h1l, 1qgu, 1qh8 - KpNase α+β subunit [Fe7MoS9 Fe8S7] – Klebsiella pneumoniae
- 1qh1 - KpNase α+β subunit + iron protein 1 [Fe7MoS9 Fe8S7]
- 1fp4 - AvNase α+β subunit (mutant) [Fe7MoS9 Fe8S8]
- 1mio - CpNase α+β subunit (mutant) [Fe7MoS9 Fe8S8] - Clostridium pasteurianum
- 4wes, 4wn9, 4wna, 5vpw, 5vq3, 5vq4 - CpNase α+β subunit [CFe7MoS9 Fe8S7 Fe2]
- 2afh, 1m1y, 1g20 - AvNase α+β subunit + iron protein 1 [Fe4S4 Fe7MoS9 Fe8S7]
- 2afi, 4wzb, 1m34, 1g21 - AvNase α+β subunit + iron protein 1 [Fe4S4 Fe7MoS9 Fe8S7] + nucleotide
- 1n2c - AvNase α+β subunit + iron protein 1 [Fe4S4 Fe7MoS9 Fe8S7] + AlF4
- 5koh, 5koj – AvNase + subunit iron protein 1 [FeS] [CFe7MoS9 Fe8S7 Fe2] – Gluconacetobacter diazotrophicus
- 3pdi – AvNase α+β subunit [Fe4S4 Fe8S9] – Azotobacter vinelandii
- Nase iron protein
- 2c8v – AvNase iron protein 1 [FeS]
- 1g1m, 1g5p, 2nip, 6o0b, 6n4k, 6n4j - AvNase iron protein 1 [Fe4S4]
- 1fp6, 1nip, 6n4m, [[6n4l] - AvNase iron protein 1 [Fe4S4] + ADP
- 1xcp, 1xd8, 1xdb, 1rw4, 1de0 - AvNase iron protein 1 (mutant) [Fe4S4]
- 6q93 - AvNase iron protein 1 [Fe4S4] + MgADP
- 1xd9 - AvNase iron protein 1 (mutant) [Fe4S4] + MgADP
- 1cp2 - CpNase iron protein 1 [Fe4S4]
- 6nzj - Nase iron protein 1 [Fe4S4] – Methanosarcina acetivorans
- 2c8v – AvNase iron protein 1 [FeS]
- Nase V-Fe protein
}}
References
- ↑ Schindelin H, Kisker C, Schlessman JL, Howard JB, Rees DC. Structure of ADP x AIF4(-)-stabilized nitrogenase complex and its implications for signal transduction. Nature. 1997 May 22;387(6631):370-6. PMID:9163420 doi:10.1038/387370a0
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Eran Hodis, David Canner, Joel L. Sussman