We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Hemeproteins
From Proteopedia
(Difference between revisions)
| Line 120: | Line 120: | ||
The <scene name='46/466465/Cv/3'>O2 molecule in the active site coordinates with the heme</scene> (PDB entry [[1dcc]]).<ref>PMID:7664080</ref> Water molecules are shown as red spheres. | The <scene name='46/466465/Cv/3'>O2 molecule in the active site coordinates with the heme</scene> (PDB entry [[1dcc]]).<ref>PMID:7664080</ref> Water molecules are shown as red spheres. | ||
| - | =Cytochrome P450 hydroxylase= | ||
| - | '''Cytochrome P450 hydroxylase''' (CPH) acts in the in-chain hydroxylation of lauric acid which is required for the development of the male organ in higher plants<ref>PMID:24798002</ref>. CPH hydroxylyzes the anti-cholesterol natural product herbosidiene<ref>PMID:25139567</ref>. | ||
| - | *<scene name='77/774654/Cv/6'>Lauric acid binding site</scene>. | ||
| - | *<scene name='77/774654/Cv/7'>Heme group binding site</scene>. | ||
| - | *<scene name='77/774654/Cv/8'>Fe coordination site</scene>. | ||
| - | *<scene name='77/774654/Cv/9'>Distances between Lauric acid oxygens and heme group Fe</scene> in Cytochrome P450 hydroxylase from ''Steptomyces avermitilis'' (PDB code [[5cwe]]). | ||
=Cytochrome c Nitrite Reductase= | =Cytochrome c Nitrite Reductase= | ||
| Line 136: | Line 130: | ||
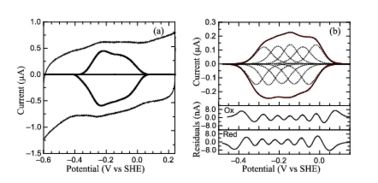
[[Image:figur5.jpg|left|378px|thumb|PFV of ''S. oneidensis'' ccNiR (a) Typical signal on a graphite electrode. (b) Baselinesubtracted non-turnover voltammogram]] | [[Image:figur5.jpg|left|378px|thumb|PFV of ''S. oneidensis'' ccNiR (a) Typical signal on a graphite electrode. (b) Baselinesubtracted non-turnover voltammogram]] | ||
The Ca<sup>2+</sup> ion within <scene name='Journal:JBIC:16/Cv/14'>conserved site</scene> is coordinated in bidentate fashion by <scene name='Journal:JBIC:16/Cv/15'>Glu205</scene>, and in monodentate fashion by the <scene name='Journal:JBIC:16/Cv/16'>Tyr206 and Lys254</scene> backbone carbonyls, and the <scene name='Journal:JBIC:16/Cv/17'>Gln256</scene> side-chain carbonyl. In the ''S. oneidensis'' structure only <scene name='Journal:JBIC:16/Cv/18'>one water molecule</scene> is assigned to the Ca<sup>2+</sup> ion in subunit B. In subunit A the difference electron density that represents this water molecule is very close to the noise level, and it is difficult to identify even one water molecule there. The <scene name='Journal:JBIC:16/Cv/14'>carbonyl side chain of Asp242 and the hydroxyl of Tyr235</scene> come near to the open calcium coordination sites, but are not within bonding distance. Instead they interact with the water molecule that is weakly coordinated to the Ca<sup>2+</sup> ion. The ccNiR calcium ions appear to play a vital role in organizing the <scene name='Journal:JBIC:16/Cv/13'>active site</scene> (as was mentioned above <font color='magenta'><b>hemes-1</b></font> are the active sites). | The Ca<sup>2+</sup> ion within <scene name='Journal:JBIC:16/Cv/14'>conserved site</scene> is coordinated in bidentate fashion by <scene name='Journal:JBIC:16/Cv/15'>Glu205</scene>, and in monodentate fashion by the <scene name='Journal:JBIC:16/Cv/16'>Tyr206 and Lys254</scene> backbone carbonyls, and the <scene name='Journal:JBIC:16/Cv/17'>Gln256</scene> side-chain carbonyl. In the ''S. oneidensis'' structure only <scene name='Journal:JBIC:16/Cv/18'>one water molecule</scene> is assigned to the Ca<sup>2+</sup> ion in subunit B. In subunit A the difference electron density that represents this water molecule is very close to the noise level, and it is difficult to identify even one water molecule there. The <scene name='Journal:JBIC:16/Cv/14'>carbonyl side chain of Asp242 and the hydroxyl of Tyr235</scene> come near to the open calcium coordination sites, but are not within bonding distance. Instead they interact with the water molecule that is weakly coordinated to the Ca<sup>2+</sup> ion. The ccNiR calcium ions appear to play a vital role in organizing the <scene name='Journal:JBIC:16/Cv/13'>active site</scene> (as was mentioned above <font color='magenta'><b>hemes-1</b></font> are the active sites). | ||
| + | |||
| + | =Cytochrome P450 hydroxylase= | ||
| + | '''Cytochrome P450 hydroxylase''' (CPH) acts in the in-chain hydroxylation of lauric acid which is required for the development of the male organ in higher plants<ref>PMID:24798002</ref>. CPH hydroxylyzes the anti-cholesterol natural product herbosidiene<ref>PMID:25139567</ref>. | ||
| + | *<scene name='77/774654/Cv/6'>Lauric acid binding site</scene>. | ||
| + | *<scene name='77/774654/Cv/7'>Heme group binding site</scene>. | ||
| + | *<scene name='77/774654/Cv/8'>Fe coordination site</scene>. | ||
| + | *<scene name='77/774654/Cv/9'>Distances between Lauric acid oxygens and heme group Fe</scene> in Cytochrome P450 hydroxylase from ''Steptomyces avermitilis'' (PDB code [[5cwe]]). | ||
=[[Hemoglobin]]= | =[[Hemoglobin]]= | ||
=[[Myoglobin]]= | =[[Myoglobin]]= | ||
Revision as of 15:20, 11 November 2019
| |||||||||||
References
- ↑ Schenkman JB, Jansson I. The many roles of cytochrome b5. Pharmacol Ther. 2003 Feb;97(2):139-52. PMID:12559387
- ↑ Rodriguez-Maranon MJ, Qiu F, Stark RE, White SP, Zhang X, Foundling SI, Rodriguez V, Schilling CL 3rd, Bunce RA, Rivera M. 13C NMR spectroscopic and X-ray crystallographic study of the role played by mitochondrial cytochrome b5 heme propionates in the electrostatic binding to cytochrome c. Biochemistry. 1996 Dec 17;35(50):16378-90. PMID:8973214 doi:10.1021/bi961895o
- ↑ Crofts AR. The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol. 2004;66:689-733. PMID:14977419 doi:http://dx.doi.org/10.1146/annurev.physiol.66.032102.150251
- ↑ Berry EA, Huang LS, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F. X-Ray Structure of Rhodobacter Capsulatus Cytochrome bc (1): Comparison with its Mitochondrial and Chloroplast Counterparts. Photosynth Res. 2004;81(3):251-75. PMID:16034531 doi:http://dx.doi.org/10.1023/B:PRES.0000036888.18223.0e
- ↑ Rajagopal BS, Wilson MT, Bendall DS, Howe CJ, Worrall JA. Structural and kinetic studies of imidazole binding to two members of the cytochrome c (6) family reveal an important role for a conserved heme pocket residue. J Biol Inorg Chem. 2011 Jan 26. PMID:21267610 doi:10.1007/s00775-011-0758-y
- ↑ Morelli X, Czjzek M, Hatchikian CE, Bornet O, Fontecilla-Camps JC, Palma NP, Moura JJ, Guerlesquin F. Structural model of the Fe-hydrogenase/cytochrome c553 complex combining transverse relaxation-optimized spectroscopy experiments and soft docking calculations. J Biol Chem. 2000 Jul 28;275(30):23204-10. PMID:10748163 doi:10.1074/jbc.M909835199
- ↑ Manole A, Kekilli D, Svistunenko DA, Wilson MT, Dobbin PS, Hough MA. Conformational control of the binding of diatomic gases to cytochrome c'. J Biol Inorg Chem. 2015 Mar 20. PMID:25792378 doi:http://dx.doi.org/10.1007/s00775-015-1253-7
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 Stelter M, Melo AM, Pereira MM, Gomes CM, Hreggvidsson GO, Hjorleifsdottir S, Saraiva LM, Teixeira M, Archer M. A Novel Type of Monoheme Cytochrome c: Biochemical and Structural Characterization at 1.23 A Resolution of Rhodothermus marinus Cytochrome c. Biochemistry. 2008 Oct 15. PMID:18855424 doi:10.1021/bi800999g
- ↑ Than ME, Hof P, Huber R, Bourenkov GP, Bartunik HD, Buse G, Soulimane T. Thermus thermophilus cytochrome-c552: A new highly thermostable cytochrome-c structure obtained by MAD phasing. J Mol Biol. 1997 Aug 29;271(4):629-44. PMID:9281430 doi:10.1006/jmbi.1997.1181
- ↑ Soares CM, Baptista AM, Pereira MM, Teixeira M. Investigation of protonatable residues in Rhodothermus marinus caa3 haem-copper oxygen reductase: comparison with Paracoccus denitrificans aa3 haem-copper oxygen reductase. J Biol Inorg Chem. 2004 Mar;9(2):124-34. Epub 2003 Dec 23. PMID:14691678 doi:10.1007/s00775-003-0509-9
- ↑ Pereira MM, Santana M, Teixeira M. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim Biophys Acta. 2001 Jun 1;1505(2-3):185-208. PMID:11334784
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 Karp, Gerald (2008). Cell and Molecular Biology (5th edition). Hoboken, NJ: John Wiley & Sons. ISBN 978-0470042175.
- ↑ Prince RC, George GN. Cytochrome f revealed. Trends Biochem Sci. 1995 Jun;20(6):217-8. PMID:7631417
- ↑ Martinez SE, Huang D, Szczepaniak A, Cramer WA, Smith JL. Crystal structure of chloroplast cytochrome f reveals a novel cytochrome fold and unexpected heme ligation. Structure. 1994 Feb 15;2(2):95-105. PMID:8081747
- ↑ Danielson PB. The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab. 2002 Dec;3(6):561-97. PMID:12369887
- ↑ Williams PA, Cosme J, Ward A, Angove HC, Matak Vinkovic D, Jhoti H. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature. 2003 Jul 24;424(6947):464-8. Epub 2003 Jul 13. PMID:12861225 doi:http://dx.doi.org/10.1038/nature01862
- ↑ Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995 Aug 25;269(5227):1069-74. PMID:7652554
- ↑ Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei MJ, Libeu CP, Mizushima T, Yamaguchi H, Tomizaki T, Tsukihara T. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998 Jun 12;280(5370):1723-9. PMID:9624044
- ↑ Atack JM, Kelly DJ. Structure, mechanism and physiological roles of bacterial cytochrome c peroxidases. Adv Microb Physiol. 2007;52:73-106. PMID:17027371 doi:http://dx.doi.org/10.1016/S0065-2911(06)52002-8
- ↑ Miller MA, Shaw A, Kraut J. 2.2 A structure of oxy-peroxidase as a model for the transient enzyme: peroxide complex. Nat Struct Biol. 1994 Aug;1(8):524-31. PMID:7664080
- ↑ 21.0 21.1 none yet
- ↑ Yang X, Wu D, Shi J, He Y, Pinot F, Grausem B, Yin C, Zhu L, Chen M, Luo Z, Liang W, Zhang D. Rice CYP703A3, a cytochrome P450 hydroxylase, is essential for development of anther cuticle and pollen exine. J Integr Plant Biol. 2014 Oct;56(10):979-94. doi: 10.1111/jipb.12212. Epub 2014, Jul 15. PMID:24798002 doi:http://dx.doi.org/10.1111/jipb.12212
- ↑ Yu D, Xu F, Shao L, Zhan J. A specific cytochrome P450 hydroxylase in herboxidiene biosynthesis. Bioorg Med Chem Lett. 2014 Sep 15;24(18):4511-4514. doi:, 10.1016/j.bmcl.2014.07.078. Epub 2014 Aug 6. PMID:25139567 doi:http://dx.doi.org/10.1016/j.bmcl.2014.07.078