TEM1-beta-Lactamase/beta-lactamase Inhibitor Protein (BLIP)
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
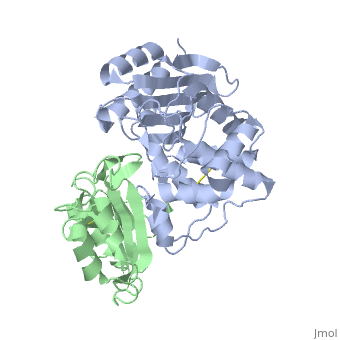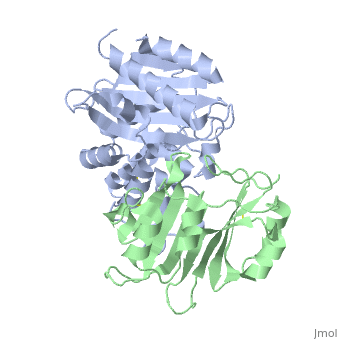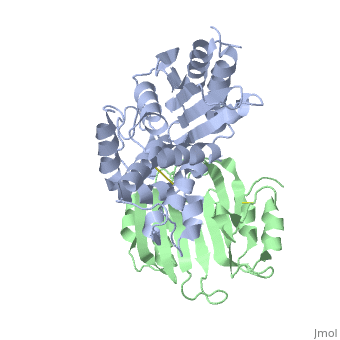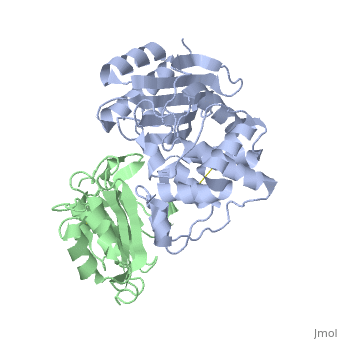
To analyze the contribution of non-alanine mutations, E104Y and Y105N in the TEM1 protein were constructed. These residues are polar and have a similar size, hence no major structural changes are expected for these mutants. The complex structure of TEM1 E104Y-Y105N (TEM1<sub>YN</sub>) with BLIP<sub>wt</sub> was solved ([[2b5r]]). The YN mutation caused only small reduction of binding energy (4.2 kJ/mol), which is slightly less then that obtained for the alanine substitutions of these residues. The <scene name='2b5r/Superpos/1'>superposition</scene> of <font color='magenta'><b>TEM1<sub>wt</sub></b></font>-<font color='black'><b>BLIP<sub>wt</sub></b></font> (yellow) complex ([[1jtg]]; <font color='magenta'><b>TEM1 colored in magenta;</b></font> <font color='black'><b>BLIP in yellow</b></font>) with <font color='lime'><b>TEM1<sub>YN</sub> (colored in lime)</b></font> - <font color='pink'><b>BLIP<sub>wt</sub> (pink)</b></font> complex ([[2b5r]]). Only a <scene name='2b5r/Superposition/12'>minor change</scene> <font color='orange'><b>(colored orange)</b></font> in <scene name='2b5r/Superpos/2'>TEM1<sub>YN</sub></scene> was observed. But, these small changes in TEM1 sequence lead to a major local <scene name='2b5r/Blip/3'>backbone rearrangement</scene> of the <scene name='2b5r/Blip/4'>hairpin loop</scene> between <font color='blue'><b>residues 46–53</b></font> in <font color='black'><b>BLIP</b></font> (yellow). This rearrangement of the <font color='red'><b>loop</b></font> is not observed in the <font color='pink'><b>unbound BLIP</b></font> structure. This shows that the rearrangement of BLIP was caused by the TEM1 mutations, resulting in a new low energy state. <scene name='2b5r/Superpos/3'>Superposition</scene> of residues in wt and mutant complexes near the BLIP 46-53 loop. TEM1 <font color='cyan'><b>wt E104 and Y105 are colored cyan</b></font>, while <font color='orange'><b>mutant E104Y and Y105N are colored orange</b></font>. BLIP residues colored in <font color='blue'><b>blue</b></font> and <font color='red'><b>red</b></font> in the <font color='blue'><b>wt</b></font> and <font color='red'><b>mutant</b></font> complexes<ref name="JMB">PMID:17070843</ref>. | To analyze the contribution of non-alanine mutations, E104Y and Y105N in the TEM1 protein were constructed. These residues are polar and have a similar size, hence no major structural changes are expected for these mutants. The complex structure of TEM1 E104Y-Y105N (TEM1<sub>YN</sub>) with BLIP<sub>wt</sub> was solved ([[2b5r]]). The YN mutation caused only small reduction of binding energy (4.2 kJ/mol), which is slightly less then that obtained for the alanine substitutions of these residues. The <scene name='2b5r/Superpos/1'>superposition</scene> of <font color='magenta'><b>TEM1<sub>wt</sub></b></font>-<font color='black'><b>BLIP<sub>wt</sub></b></font> (yellow) complex ([[1jtg]]; <font color='magenta'><b>TEM1 colored in magenta;</b></font> <font color='black'><b>BLIP in yellow</b></font>) with <font color='lime'><b>TEM1<sub>YN</sub> (colored in lime)</b></font> - <font color='pink'><b>BLIP<sub>wt</sub> (pink)</b></font> complex ([[2b5r]]). Only a <scene name='2b5r/Superposition/12'>minor change</scene> <font color='orange'><b>(colored orange)</b></font> in <scene name='2b5r/Superpos/2'>TEM1<sub>YN</sub></scene> was observed. But, these small changes in TEM1 sequence lead to a major local <scene name='2b5r/Blip/3'>backbone rearrangement</scene> of the <scene name='2b5r/Blip/4'>hairpin loop</scene> between <font color='blue'><b>residues 46–53</b></font> in <font color='black'><b>BLIP</b></font> (yellow). This rearrangement of the <font color='red'><b>loop</b></font> is not observed in the <font color='pink'><b>unbound BLIP</b></font> structure. This shows that the rearrangement of BLIP was caused by the TEM1 mutations, resulting in a new low energy state. <scene name='2b5r/Superpos/3'>Superposition</scene> of residues in wt and mutant complexes near the BLIP 46-53 loop. TEM1 <font color='cyan'><b>wt E104 and Y105 are colored cyan</b></font>, while <font color='orange'><b>mutant E104Y and Y105N are colored orange</b></font>. BLIP residues colored in <font color='blue'><b>blue</b></font> and <font color='red'><b>red</b></font> in the <font color='blue'><b>wt</b></font> and <font color='red'><b>mutant</b></font> complexes<ref name="JMB">PMID:17070843</ref>. | ||
| - | + | See also [[Journal:Structure:1|Promiscuous Protein Binding as a Function of Protein Stability]] | |
{{Clear}} | {{Clear}} | ||
</StructureSection> | </StructureSection> | ||
Revision as of 14:02, 12 November 2019
| |||||||||||
3D structures of β-lactamase inhibitor protein
Updated on 12-November-2019
References
- ↑ 1.0 1.1 1.2 Strynadka NC, Jensen SE, Johns K, Blanchard H, Page M, Matagne A, Frere JM, James MN. Structural and kinetic characterization of a beta-lactamase-inhibitor protein. Nature. 1994 Apr 14;368(6472):657-60. PMID:8145854 doi:http://dx.doi.org/10.1038/368657a0
- ↑ 2.0 2.1 Reichmann D, Rahat O, Albeck S, Meged R, Dym O, Schreiber G. The modular architecture of protein-protein binding interfaces. Proc Natl Acad Sci U S A. 2005 Jan 4;102(1):57-62. Epub 2004 Dec 23. PMID:15618400
- ↑ Reichmann D, Cohen M, Abramovich R, Dym O, Lim D, Strynadka NC, Schreiber G. Binding hot spots in the TEM1-BLIP interface in light of its modular architecture. J Mol Biol. 2007 Jan 19;365(3):663-79. Epub 2006 Oct 3. PMID:17070843 doi:10.1016/j.jmb.2006.09.076
Proteopedia Page Contributors and Editors (what is this?)
Categories: Topic Page | Escherichia coli | Protein complex | Streptomyces clavuligerus | ISPC, Israel Structural Proteomics Center. | Reichman, D. | Schreiber, G. | Beta-lactamase inhibitor protein | Blip | ISPC | Israel Structural Proteomics Center | Protein-protein complex | Structural genomic | Tem-1 beta-lactamase | Beta-lactamase | Albeck, S. | Dym, O. | Meged, R. | Rahat, O. | Beta-2 lactamase inhibitor protein | Jelsch, C. | Masson, J M. | Mourey, L. | Samama, J P. | Hydrolase