This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Phosphodiesterase
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
== Structural highlights == | == Structural highlights == | ||
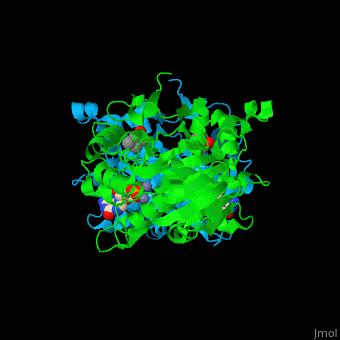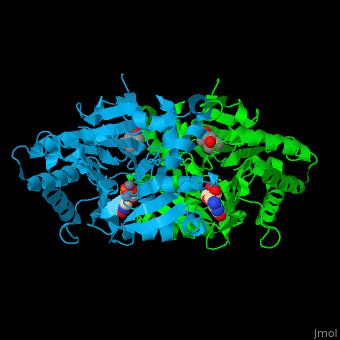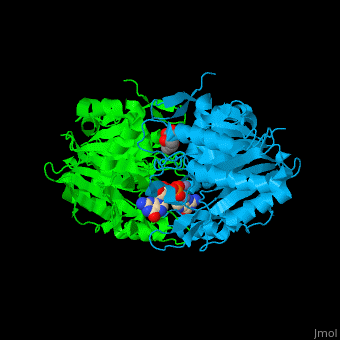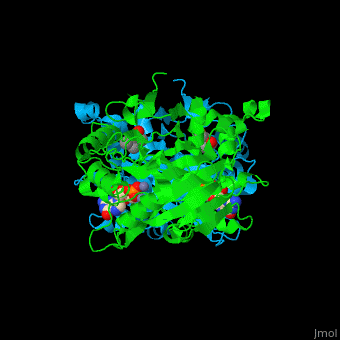
The yeast PDE 1 <scene name='47/478801/Cv/3'>cGMP binding pocket</scene> is located between 2 monomers and contains <scene name='47/478801/Cv/5'>2 Zn+2 ions</scene><ref>PMID:25050706</ref>. Water molecules shown as red spheres. | The yeast PDE 1 <scene name='47/478801/Cv/3'>cGMP binding pocket</scene> is located between 2 monomers and contains <scene name='47/478801/Cv/5'>2 Zn+2 ions</scene><ref>PMID:25050706</ref>. Water molecules shown as red spheres. | ||
| + | |||
| + | == 3D Structures of phosphodiesterase== | ||
| + | [[Phosphodiesterase 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
| Line 42: | Line 45: | ||
**[[1ksg]], [[1ksh]], [[1ksj]] – hPDE δ subunit + ARF-like protein - human<BR /> | **[[1ksg]], [[1ksh]], [[1ksj]] – hPDE δ subunit + ARF-like protein - human<BR /> | ||
| - | **[[2ju4]] - | + | **[[2ju4]] - bPDE γ subunit – bovine - NMR<BR /> |
| + | **[[6mzb]] - bPDE α,β,γ subunits – Cryo EM<br /> | ||
**[[4kie]], [[4lyk]] - EcPDE EAL domain – ''Escherichia coli''<br /> | **[[4kie]], [[4lyk]] - EcPDE EAL domain – ''Escherichia coli''<br /> | ||
**[[4lj3]] - EcPDE EAL domain + GMP<br /> | **[[4lj3]] - EcPDE EAL domain + GMP<br /> | ||
| Line 67: | Line 71: | ||
**[[1f0j]] – mPDE 2a regulatory domain (mutant) – mouse<BR /> | **[[1f0j]] – mPDE 2a regulatory domain (mutant) – mouse<BR /> | ||
**[[1z1l]], [[3ibj]], [[3itm]] - hPDE 2a catalytic domain<BR /> | **[[1z1l]], [[3ibj]], [[3itm]] - hPDE 2a catalytic domain<BR /> | ||
| - | **[[3itu]], [[4htx]], [[4htz]], [[4jib]], [[4d08]], [[4d09]], [[5xkm]], [[5vp0]], [[5vp1]], [[5u7l]], [[5u7k]], [[5u7j]], [[5u7i]], [[5u7d]], [[5u00]], [[5tzz]], [[5tzx]], [[5tzw]], [[5tzh]], [[5tzc]], [[5tza]], [[5tz3]], [[6ezf]], [[6c7j]], [[6c7i]], [[6c7g]], [[6c7f]], [[6c7e]], [[6c7d]], [[6b98]], [[6b97]], [[6b96]], [[4c1i]] - hPDE 2a catalytic domain + inhibitor<br /> | + | **[[3itu]], [[4htx]], [[4htz]], [[4jib]], [[4d08]], [[4d09]], [[5xkm]], [[5vp0]], [[5vp1]], [[5u7l]], [[5u7k]], [[5u7j]], [[5u7i]], [[5u7d]], [[5u00]], [[5tzz]], [[5tzx]], [[5tzw]], [[5tzh]], [[5tzc]], [[5tza]], [[5tz3]], [[6ezf]], [[6c7j]], [[6c7i]], [[6c7g]], [[6c7f]], [[6c7e]], [[6c7d]], [[6b98]], [[6b97]], [[6b96]], [[4c1i]], [[6cyd]], [[6cyc]], [[6cyb]], [[6blf]] - hPDE 2a catalytic domain + inhibitor<br /> |
| + | **[[1f0j]] – mPDE 2a regulatory domain (mutant) – mouse<BR /> | ||
| + | **[[6cpu]] - CaPDE 2 – ''Candida albicans''<br /> | ||
| + | **[[6cpt]] - CaPDE 2 + IBMX<br /> | ||
*PDE 2H or RNA 2’,3’-cyclic PDE | *PDE 2H or RNA 2’,3’-cyclic PDE | ||
| Line 80: | Line 87: | ||
**[[1so2]], [[1soj]] – hPDE 3b catalytic domain + inhibitor<br /> | **[[1so2]], [[1soj]] – hPDE 3b catalytic domain + inhibitor<br /> | ||
| + | **[[5z7c]] – PDE 3 (mutant) – ''Vibrio cholerae'' <br /> | ||
*PDE 4a | *PDE 4a | ||
**[[3i8v]], [[3tvx]] – hPDE 4a catalytic domain + inhibitor<br /> | **[[3i8v]], [[3tvx]] – hPDE 4a catalytic domain + inhibitor<br /> | ||
| + | **[[6fg5]], [[6ezu]] - PDE 4a residues 230-597 – ''Schistosoma mansoni''<br /> | ||
*PDE 4b or cAMP-specific 3’,5’-cyclic PDE | *PDE 4b or cAMP-specific 3’,5’-cyclic PDE | ||
| Line 106: | Line 115: | ||
**[[1ptw]], [[1tb7]] - hPDE 4d catalytic domain + AMP<BR /> | **[[1ptw]], [[1tb7]] - hPDE 4d catalytic domain + AMP<BR /> | ||
**[[2pw3]] - hPDE 4d catalytic domain (mutant) + AMP<BR /> | **[[2pw3]] - hPDE 4d catalytic domain (mutant) + AMP<BR /> | ||
| - | **[[1y2b]], [[1y2c]], [[1y2d]], [[1y2e]], [[1y2k]], [[1zkn]], [[2fm0]], [[2fm5]], [[2qyn]], [[3k4s]], [[3sl3]], [[3sl4]], [[3sl5]], [[3sl6]], [[3sl8]], [[3v9b]], [[5wqa]], [[5tkb]], [[5k32]], [[5k1i]], [[4wcu]], [[4w1o]], [[4ogb]] - hPDE 4d catalytic domain + inhibitor<br /> | + | **[[1y2b]], [[1y2c]], [[1y2d]], [[1y2e]], [[1y2k]], [[1zkn]], [[2fm0]], [[2fm5]], [[2qyn]], [[3k4s]], [[3sl3]], [[3sl4]], [[3sl5]], [[3sl6]], [[3sl8]], [[3v9b]], [[5wqa]], [[5tkb]], [[5k32]], [[5k1i]], [[4wcu]], [[4w1o]], [[4ogb]], [[6fdc]], [[6f8x]], [[6f8w]], [[6f8v]], [[6f8u]], [[6f8t]], [[6f8r]], [[6f6u]], [[5wh6]], [[5wh5]], [[5lbo]], [[6rcw]], [[6inm]], [[6ink]], [[6ind]], [[6imt]], [[6imr]], [[6imo]], [[6imd]], [[6imi]], [[6imb]], [[6im6]], [[6hwo]], [[6fw3]], [[6ftw]], [[6fta]], [[6ft0]], [[6fet]], [[6feb]], [[6fe7]], [[6fdi]], [[6boj]] - hPDE 4d catalytic domain + inhibitor<br /> |
| + | **[[6njj]], [[6nji]], [[6njh]] - hPDE 4d catalytic domain (mutant) + inhibitor<br /> | ||
**[[3iad]], [[3g4g]], [[3g4i]], [[3g58]] - hPDE 4d catalytic domain + modulator | **[[3iad]], [[3g4g]], [[3g4i]], [[3g58]] - hPDE 4d catalytic domain + modulator | ||
| Line 113: | Line 123: | ||
**[[2h40]] - hPDE 5a catalytic domain <BR /> | **[[2h40]] - hPDE 5a catalytic domain <BR /> | ||
**[[1t9r]] - hPDE 5 catalytic domain<BR /> | **[[1t9r]] - hPDE 5 catalytic domain<BR /> | ||
| - | **[[2k31]] - mPDE 5a cGMP-binding domain – NMR<BR /> | ||
**[[3lfv]], [[3mf0]], [[2xss]] - hPDE 5a GAF domain<BR /> | **[[3lfv]], [[3mf0]], [[2xss]] - hPDE 5a GAF domain<BR /> | ||
**[[1rkp]], [[3tgg]] – hPDE 5a1 catalytic domain (mutant) + inhibitor<br /> | **[[1rkp]], [[3tgg]] – hPDE 5a1 catalytic domain (mutant) + inhibitor<br /> | ||
| - | **[[2chm]], [[4oex]], [[4oew]], [[4g2w]], [[4g2y]], [[4i9z]], [[4ia0]], [[4md6]], [[5jo3]] - hPDE 5a catalytic domain + inhibitor<br /> | + | **[[2chm]], [[4oex]], [[4oew]], [[4g2w]], [[4g2y]], [[4i9z]], [[4ia0]], [[4md6]], [[5jo3]], [[6acb]], [[5zz2]] - hPDE 5a catalytic domain + inhibitor<br /> |
**[[2h44]], [[3bjc]], [[3hc8]], [[3hdz]], [[3shy]], [[3shz]], [[3sie]], [[3tge]] - hPDE 5a1 catalytic domain + inhibitor<br /> | **[[2h44]], [[3bjc]], [[3hc8]], [[3hdz]], [[3shy]], [[3shz]], [[3sie]], [[3tge]] - hPDE 5a1 catalytic domain + inhibitor<br /> | ||
**[[1udt]], [[1udu]], [[1uho]], [[1tbf]], [[1xoz]], [[1xp0]], [[2h42]], [[3b2r]] - hPDE 5 catalytic domain + drug<br /> | **[[1udt]], [[1udu]], [[1uho]], [[1tbf]], [[1xoz]], [[1xp0]], [[2h42]], [[3b2r]] - hPDE 5 catalytic domain + drug<br /> | ||
| Line 122: | Line 131: | ||
**[[3jwr]] - hPDE 5/hPDE 6 catalytic domain + hPDE γ subunit peptide + inhibitor<br /> | **[[3jwr]] - hPDE 5/hPDE 6 catalytic domain + hPDE γ subunit peptide + inhibitor<br /> | ||
**[[1t9s]] - hPDE 5 catalytic domain + GMP<br /> | **[[1t9s]] - hPDE 5 catalytic domain + GMP<br /> | ||
| + | **[[2k31]] - mPDE 5a cGMP-binding domain – NMR<BR /> | ||
**[[5w10]] - PDE + cAMP – ''Leptospira interrogans''<br /> | **[[5w10]] - PDE + cAMP – ''Leptospira interrogans''<br /> | ||
| Line 147: | Line 157: | ||
*PDE 9 | *PDE 9 | ||
| - | **[[2hd1]], [[2yy2]], [[3jsi]], [[3jsw]], [[3k3e]], [[3k3h]], [[4e90]], [[4g2j]], [[4g2l]], [[4gh6]] - hPDE 6d δ subunit + RPGR<br /> | + | **[[2hd1]], [[2yy2]], [[3jsi]], [[3jsw]], [[3k3e]], [[3k3h]], [[4e90]], [[4g2j]], [[4g2l]], [[4gh6]],[[4y8c]], [[4y87]], [[4y86]], [[6a3n]] - hPDE 6d δ subunit + RPGR<br /> |
**[[3n3z]], [[3qi3]], [[3qi4]] - hPDE 9a catalytic domain (mutant) + inhibitor<br /> | **[[3n3z]], [[3qi3]], [[3qi4]] - hPDE 9a catalytic domain (mutant) + inhibitor<br /> | ||
**[[3dy8]], [[3dyl]], [[3dyn]], [[3dyq]], [[3dys]] - hPDE 9 catalytic domain + GMP<br /> | **[[3dy8]], [[3dyl]], [[3dyn]], [[3dyq]], [[3dys]] - hPDE 9 catalytic domain + GMP<br /> | ||
| Line 155: | Line 165: | ||
*PDE 10 or cAMP and cAMP-inhibited cGMP 3’,5’-cyclic PDE | *PDE 10 or cAMP and cAMP-inhibited cGMP 3’,5’-cyclic PDE | ||
| - | **[[2o8h]] – rPDE 10a catalytic domain<BR /> | ||
**[[2oup]] - hPDE 10a catalytic domain<BR /> | **[[2oup]] - hPDE 10a catalytic domain<BR /> | ||
**[[2zmf]] – hPDE 10a C terminal<BR /> | **[[2zmf]] – hPDE 10a C terminal<BR /> | ||
**[[2ous]], [[2ouv]] - hPDE 10a2 catalytic domain (mutant) <BR /> | **[[2ous]], [[2ouv]] - hPDE 10a2 catalytic domain (mutant) <BR /> | ||
| - | + | **[[2qyk]], [[3lxg]], [[2y0j]], [[3qpn]], [[3qpo]], [[3qpp]], [[3sn7]], [[3sni]], [[3snl]], [[3wi2]], [[4ael]], [[4ajd]], [[4ajf]], [[4ajg]], [[4ajm]], [[4ddl]], [[4fcb]], [[4fcd]], [[4heu]], [[4hf4]], [[4lkq]], [[4llj]], [[4llk]], [[4llp]], [[4llx]], [[4lm0]], [[4lm1]], [[4lm2]], [[4lm3]], [[4lm4]], [[4muw]], [[4mvh]], [[4yqh]], [[4ys7]], [[3ws8]], [[3ws9]], [[3wyk]], [[3wyl]], [[3wym]], [[4mrw]], [[4mrz]], [[4ms0]], [[4msa]], [[4msc]], [[4mse]], [[4msh]], [[4msn]], [[5uwf]], [[5k9r]], [[5i2r]], [[5edi]], [[5edh]], [[5edg]], [[5ede]], [[5dh5]], [[5dh4]], [[5c2h]], [[5c2e]], [[5c2a]], [[5c29]], [[5c28]], [[5c1w]], [[5b4k]], [[5b4l]], [[5axq]], [[5axp]], [[4zo5]], [[4xy2]], [[4wn1]], [[4tpp]], [[4tpm]], [[4phw]], [[4p1r]], [[4p0n]], [[5xuj]], [[5xui]], [[6msc]], [[6msa]], [[6ke0]], [[6kdz]], [[6kdx]], [[6iji]], [[6ijh]], [[5znl]] - hPDE 10a catalytic domain + inhibitor<br /> | |
| - | **[[2qyk]], [[3lxg]], [[2y0j]], [[3qpn]], [[3qpo]], [[3qpp]], [[3sn7]], [[3sni]], [[3snl]], [[3wi2]], [[4ael]], [[4ajd]], [[4ajf]], [[4ajg]], [[4ajm]], [[4ddl]], [[4fcb]], [[4fcd]], [[4heu]], [[4hf4]], [[4lkq]], [[4llj]], [[4llk]], [[4llp]], [[4llx]], [[4lm0]], [[4lm1]], [[4lm2]], [[4lm3]], [[4lm4]], [[4muw]], [[4mvh]], [[4yqh]], [[4ys7]], [[3ws8]], [[3ws9]], [[3wyk]], [[3wyl]], [[3wym]], [[4mrw]], [[4mrz]], [[4ms0]], [[4msa]], [[4msc]], [[4mse]], [[4msh]], [[4msn]], [[5uwf]], [[5k9r]], [[5i2r]], [[5edi]], [[5edh]], [[5edg]], [[5ede]], [[5dh5]], [[5dh4]], [[5c2h]], [[5c2e]], [[5c2a]], [[5c29]], [[5c28]], [[5c1w]], [[5b4k]], [[5b4l]], [[5axq]], [[5axp]], [[4zo5]], [[4xy2]], [[4wn1]], [[4tpp]], [[4tpm]], [[4phw]], [[4p1r]], [[4p0n]], [[5xuj]], [[5xui]] - hPDE 10a catalytic domain + inhibitor<br /> | + | |
**[[2wey]], [[3hqw]], [[3hqy]], [[3hqz]], [[3hr1]], [[3ui7]], [[3uuo]], [[4bbx]], [[4dff]] - hPDE 10a catalytic domain + schizophrenia drug<br /> | **[[2wey]], [[3hqw]], [[3hqy]], [[3hqz]], [[3hr1]], [[3ui7]], [[3uuo]], [[4bbx]], [[4dff]] - hPDE 10a catalytic domain + schizophrenia drug<br /> | ||
**[[2oun]] - hPDE 10a2 catalytic domain + AMP<BR /> | **[[2oun]] - hPDE 10a2 catalytic domain + AMP<BR /> | ||
**[[2our]], [[2ouy]] - hPDE 10a2 catalytic domain (mutant) + AMP<BR /> | **[[2our]], [[2ouy]] - hPDE 10a2 catalytic domain (mutant) + AMP<BR /> | ||
**[[2ouq]] - hPDE 10a2 catalytic domain + GMP<BR /> | **[[2ouq]] - hPDE 10a2 catalytic domain + GMP<BR /> | ||
| - | **[[2ouu]] - hPDE 10a2 catalytic domain (mutant) + GMP | + | **[[2ouu]] - hPDE 10a2 catalytic domain (mutant) + GMP<br /> |
| + | **[[2o8h]] – rPDE 10a catalytic domain<BR /> | ||
| + | **[[6k9u]], [[2ovv]], [[2ovy]] - rPDE 10a catalytic domain + inhibitor<br /> | ||
*PDE 12 | *PDE 12 | ||
| Line 175: | Line 185: | ||
**[[4i15]] – TbPDEb1 catalytic domain – ''Trypanosoma brucei''<br /> | **[[4i15]] – TbPDEb1 catalytic domain – ''Trypanosoma brucei''<br /> | ||
| - | **[[5l9h]], [[5l8y]], [[5l8c]], [[5g5v]], [[5g57]], [[5g2b]], [[4phl]] – TbPDEb1 catalytic domain + inhibitor<br /> | + | **[[5l9h]], [[5l8y]], [[5l8c]], [[5g5v]], [[5g57]], [[5g2b]], [[4phl]], [[6rgk]], [[6rfw]], [[6rfn]], [[6rb6]], [[6gxq]], [[6fv9]], [[6ftm]], [[6frd]], [[6fe3]], [[6fdx]], [[6fdw]], [[6fds]] – TbPDEb1 catalytic domain + inhibitor<br /> |
*PDE b2 | *PDE b2 | ||
| Line 184: | Line 194: | ||
**[[5v1m]] - hPDE + UMP <br /> | **[[5v1m]] - hPDE + UMP <br /> | ||
| + | **[[6d31]] - hPDE + AMP <br /> | ||
| + | **[[6d30]], [[6d2z]] - hPDE (mutant) + RNA <br /> | ||
**[[5uqj]] - yPDE <br /> | **[[5uqj]] - yPDE <br /> | ||
| Line 221: | Line 233: | ||
*Tyrosyl-DNA PDE | *Tyrosyl-DNA PDE | ||
| - | **[[ | + | **[[1jy1]], [[1qzq]] – hPDE1 catalytic domain<BR /> |
| - | **[[ | + | **[[1mu7]] - hPDE1 catalytic domain (mutant) + WO4<BR /> |
| - | **[[ | + | **[[1mu9]] - hPDE1 catalytic domain (mutant) + VO4<BR /> |
| + | **[[1nop]], [[1rff]], [[1rfi]], [[1rg1]], [[1rg2]], [[1rgt]] - hPDE1 catalytic domain (mutant) + DNA + topoisomerase peptide + VO4<BR /> | ||
| + | **[[1rgu]], [[1rh0]] - hPDE1 catalytic domain (mutant) + polynucleotide + neurohormone<br /> | ||
| + | **[[6n19]], [[6n17]], [[6n0r]], [[6n0o]], [[6n0n]], [[6n0d]], [[6mz0]], [[6myz]], [[6mj5]], [[6djj]], [[6dji]], [[6djh]], [[6djg]], [[6djf]], [[6dje]], [[6djd]], [[6dim]], [[6dih]], [[6die]], [[6dhu]] - hPDE catalytic domain + inhibitor<br /> | ||
| + | **[[5j3s]], [[5j3p]] - hPDE catalytic domain (mutant) + inhibitor<br /> | ||
| + | **[[5nwa]], [[5nw9]] – hPDE1 residues 1-148 + DNA <br /> | ||
| + | **[[5j3s]], [[5j3p]] - hPDE1 catalytic domain (mutant) + inhibitor<br /> | ||
**[[5inm]] - mPDE catalytic domain <br /> | **[[5inm]] - mPDE catalytic domain <br /> | ||
**[[5inn]] - mPDE catalytic domain (mutant) <br /> | **[[5inn]] - mPDE catalytic domain (mutant) <br /> | ||
| - | **[[4f1i]], [[4fva]], [[4gew]] – PDE – ''Caenorhabditis elegans''<br /> | ||
| - | **[[1mu7]] - hPDE1 residues 149-608 (mutant) + WO4<BR /> | ||
| - | **[[1mu9]] - hPDE1 residues 149-608 (mutant) + VO4<BR /> | ||
| - | **[[1nop]], [[1rff]], [[1rfi]], [[1rg1]], [[1rg2]], [[1rgt]] - hPDE1 residues 149-608 (mutant) + DNA + topoisomerase peptide + VO4<BR /> | ||
| - | **[[1rgu]], [[1rh0]] - hPDE1 residues 149-608 (mutant) + polynucleotide + neurohormone<br /> | ||
| - | **[[5nwa]], [[5nw9]] – hPDE1 + DNA <br /> | ||
| - | **[[4f1h]], [[4fpv]] – zPDE + DNA – zebra fish<br /> | ||
**[[4gz0]], [[4gz1]], [[4gz2]], [[4puq]], [[5inq]], [[5inp]], [[5ino]], [[5inl]], [[5ink]], [[5ht2]] – mPDE catalytic domain + DNA<br /> | **[[4gz0]], [[4gz1]], [[4gz2]], [[4puq]], [[5inq]], [[5inp]], [[5ino]], [[5inl]], [[5ink]], [[5ht2]] – mPDE catalytic domain + DNA<br /> | ||
**[[5tvq]] – mPDE catalytic domain + SUMO-2<br /> | **[[5tvq]] – mPDE catalytic domain + SUMO-2<br /> | ||
**[[5tvp]] – mPDE catalytic domain + SUMO-2 + DNA<br /> | **[[5tvp]] – mPDE catalytic domain + SUMO-2 + DNA<br /> | ||
**[[5j42]], [[5j3z]] - mPDE catalytic domain (mutant) + inhibitor<br /> | **[[5j42]], [[5j3z]] - mPDE catalytic domain (mutant) + inhibitor<br /> | ||
| - | **[[ | + | **[[4f1h]], [[4fpv]] – zPDE + DNA – zebra fish<br /> |
**[[6ca4]] - zPDE catalytic domain (mutant) + inhibitor <br /> | **[[6ca4]] - zPDE catalytic domain (mutant) + inhibitor <br /> | ||
| + | **[[1q32]] – yPDE <BR /> | ||
| + | **[[3sq3]], [[3sq5]], [[3sq7]], [[3sq8]] – yPDE (mutant) <BR /> | ||
| + | **[[4f1i]], [[4fva]], [[4gew]] – PDE – ''Caenorhabditis elegans''<br /> | ||
*Glycerophosphodiester PDE | *Glycerophosphodiester PDE | ||
Revision as of 10:42, 17 November 2019
| |||||||||||
3D Structures of phosphodiesterase
Updated on 17-November-2019
References
- ↑ Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007 May 1;115(17):2331-9. Epub 2007 Apr 16. PMID:17438150 doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.106.676809
- ↑ Whalin ME, Scammell JG, Strada SJ, Thompson WJ. Phosphodiesterase II, the cGMP-activatable cyclic nucleotide phosphodiesterase, regulates cyclic AMP metabolism in PC12 cells. Mol Pharmacol. 1991 Jun;39(6):711-7. PMID:1646946
- ↑ Tilley DG, Maurice DH. Vascular smooth muscle cell phosphodiesterase (PDE) 3 and PDE4 activities and levels are regulated by cyclic AMP in vivo. Mol Pharmacol. 2002 Sep;62(3):497-506. PMID:12181425
- ↑ Morin F, Lugnier C, Kameni J, Voisin P. Expression and role of phosphodiesterase 6 in the chicken pineal gland. J Neurochem. 2001 Jul;78(1):88-99. PMID:11432976
- ↑ Redondo M, Brea J, Perez DI, Soteras I, Val C, Perez C, Morales-Garcia JA, Alonso-Gil S, Paul-Fernandez N, Martin-Alvarez R, Cadavid MI, Loza MI, Perez-Castillo A, Mengod G, Campillo NE, Martinez A, Gil C. Effect of phosphodiesterase 7 (PDE7) inhibitors in experimental autoimmune encephalomyelitis mice. Discovery of a new chemically diverse family of compounds. J Med Chem. 2012 Apr 12;55(7):3274-84. doi: 10.1021/jm201720d. Epub 2012 Mar 16. PMID:22385507 doi:http://dx.doi.org/10.1021/jm201720d
- ↑ Dong H, Osmanova V, Epstein PM, Brocke S. Phosphodiesterase 8 (PDE8) regulates chemotaxis of activated lymphocytes. Biochem Biophys Res Commun. 2006 Jun 30;345(2):713-9. Epub 2006 May 3. PMID:16696947 doi:http://dx.doi.org/10.1016/j.bbrc.2006.04.143
- ↑ Singh N, Patra S. Phosphodiesterase 9: insights from protein structure and role in therapeutics. Life Sci. 2014 Jun 13;106(1-2):1-11. doi: 10.1016/j.lfs.2014.04.007. Epub 2014, Apr 16. PMID:24746902 doi:http://dx.doi.org/10.1016/j.lfs.2014.04.007
- ↑ Qiu Y, Yanase T, Hu H, Tanaka T, Nishi Y, Liu M, Sueishi K, Sawamura T, Nawata H. Dihydrotestosterone suppresses foam cell formation and attenuates atherosclerosis development. Endocrinology. 2010 Jul;151(7):3307-16. doi: 10.1210/en.2009-1268. Epub 2010 Apr , 28. PMID:20427482 doi:http://dx.doi.org/10.1210/en.2009-1268
- ↑ Wood ER, Bledsoe R, Chai J, Daka P, Deng H, Ding Y, Harris-Gurley S, Kryn LH, Nartey E, Nichols J, Nolte RT, Prabhu N, Rise C, Sheahan T, Shotwell JB, Smith D, Tai V, Taylor JD, Tomberlin G, Wang L, Wisely B, You S, Xia B, Dickson H. The Role of Phosphodiesterase 12 (PDE12) as a Negative Regulator of the Innate Immune Response and the Discovery of Antiviral Inhibitors. J Biol Chem. 2015 Jun 8. pii: jbc.M115.653113. PMID:26055709 doi:http://dx.doi.org/10.1074/jbc.M115.653113
- ↑ Mroczek S, Krwawicz J, Kutner J, Lazniewski M, Kucinski I, Ginalski K, Dziembowski A. C16orf57, a gene mutated in poikiloderma with neutropenia, encodes a putative phosphodiesterase responsible for the U6 snRNA 3' end modification. Genes Dev. 2012 Sep 1;26(17):1911-25. doi: 10.1101/gad.193169.112. Epub 2012 Aug , 16. PMID:22899009 doi:10.1101/gad.193169.112
- ↑ Schmidt AJ, Ryjenkov DA, Gomelsky M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol. 2005 Jul;187(14):4774-81. PMID:15995192 doi:http://dx.doi.org/187/14/4774
- ↑ Jin SL, Ding SL, Lin SC. Phosphodiesterase 4 and its inhibitors in inflammatory diseases. Chang Gung Med J. 2012 May-Jun;35(3):197-210. PMID:22735051
- ↑ Reffelmann T, Kloner RA. Cardiovascular effects of phosphodiesterase 5 inhibitors. Curr Pharm Des. 2006;12(27):3485-94. PMID:17017941
- ↑ Garcia AM, Redondo M, Martinez A, Gil C. Phosphodiesterase 10 inhibitors: new disease modifying drugs for Parkinson's disease? Curr Med Chem. 2014 Apr;21(10):1171-87. PMID:24372206
- ↑ Tian Y, Cui W, Huang M, Robinson H, Wan Y, Wang Y, Ke H. Dual specificity and novel structural folding of yeast phosphodiesterase-1 for hydrolysis of second messengers cyclic adenosine and guanosine 3',5'-monophosphate. Biochemistry. 2014 Aug 5;53(30):4938-45. doi: 10.1021/bi500406h. Epub 2014 Jul, 22. PMID:25050706 doi:http://dx.doi.org/10.1021/bi500406h
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Rick H. Cote