We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1559
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
[[Image:6ojtsecondarystructure.png|600 px]] | [[Image:6ojtsecondarystructure.png|600 px]] | ||
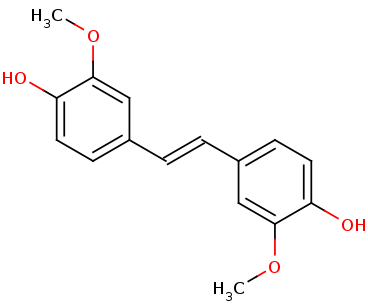
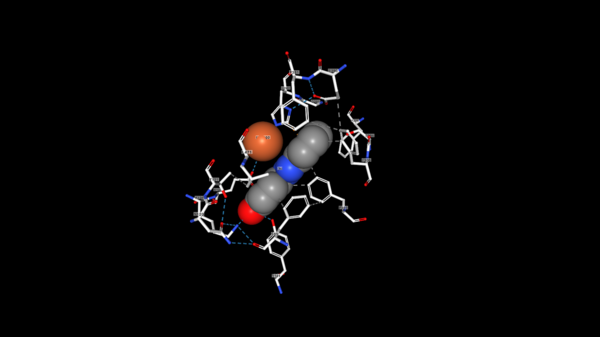
| - | The tertiary structure creates a<scene name='82/823083/Aminobindingpocket/1'> binding pocket of amino acids</scene> that are important to the active site. His282 provides pi-stacking, Phe305 provides Hydrophobic contacts, and Tyr101 provides Hydrogen bonding. The tertiary structure also allows the NSL ligand to interact using its 4-hydroxy with the catalytic triad. LsdA can only cleave 4-hydroxystilbenes. | + | The tertiary structure creates a<scene name='82/823083/Aminobindingpocket/1'> binding pocket of amino acids</scene> that are important to the active site. His282 provides pi-stacking, Phe305 provides Hydrophobic contacts, and Tyr101 provides Hydrogen bonding. The tertiary structure also allows the NSL ligand to interact using its 4-hydroxy with the catalytic triad. LsdA can only cleave 4-hydroxystilbenes. The photo below shows the NSL ligand interacting in the binding pocket, and it also shows the orange heme interacting with the amino group on the ligand. |
| - | [[Image: | + | [[Image:NSL_ligand.png|600 px]] |
== Energy Transformation == | == Energy Transformation == | ||
Revision as of 21:13, 29 November 2019
| This Sandbox is Reserved from Aug 26 through Dec 12, 2019 for use in the course CHEM 351 Biochemistry taught by Bonnie_Hall at the Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1556 through Sandbox Reserved 1575. |
To get started:
More help: Help:Editing |
Overview
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644