We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1568
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_BHall_Chem351_F19}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_BHall_Chem351_F19}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | ==Lignostilbene-α,ß-dioxygenase A structural features and important functional residues== | + | == '''Lignostilbene-α,ß-dioxygenase A structural features and important functional residues''' == |
<StructureSection load='6ojt' size='420' side='right' caption='LsdA phenylazophenol complex' scene=''> | <StructureSection load='6ojt' size='420' side='right' caption='LsdA phenylazophenol complex' scene=''> | ||
You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
| - | == | + | == '''Functions and Biological Relevance''' == |
Lignostilbene-α,ß-dioxygenase A (LsdA) from the bacterium ''Sphingomonas paucimobilis'' TMY1009 is a nonheme iron oxygenase that catalyzes the cleavage via oxygenolytic fission of lignostilbene, a compound arising in lignin transformation, to two vanillin molecules (see images below). Lignin is a common component of biomass from industry that scientists are interested in finding ways to break down to simpler and useful materials such as biofuels and commodity chemicals. Though natural occurring lignostilbenoids are rare, they are a very comm on byproduct of industry due to condensation reactions. Other lignin-based stilbenes are thought to be produces from bacterial catabolic processing of diaryl propane and phenylcoumarane <ref>PMID 31292192</ref>. | Lignostilbene-α,ß-dioxygenase A (LsdA) from the bacterium ''Sphingomonas paucimobilis'' TMY1009 is a nonheme iron oxygenase that catalyzes the cleavage via oxygenolytic fission of lignostilbene, a compound arising in lignin transformation, to two vanillin molecules (see images below). Lignin is a common component of biomass from industry that scientists are interested in finding ways to break down to simpler and useful materials such as biofuels and commodity chemicals. Though natural occurring lignostilbenoids are rare, they are a very comm on byproduct of industry due to condensation reactions. Other lignin-based stilbenes are thought to be produces from bacterial catabolic processing of diaryl propane and phenylcoumarane <ref>PMID 31292192</ref>. | ||
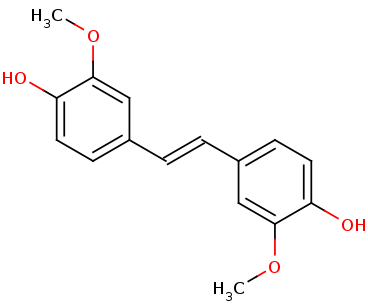
| - | == Lignostilbene == | + | == '''Lignostilbene''' == |
[[Image:lignostilbene.png]] | [[Image:lignostilbene.png]] | ||
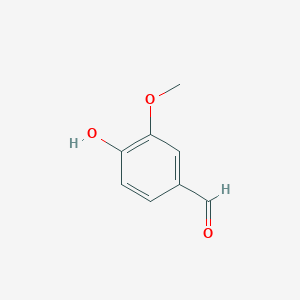
| - | == Vanillin == | + | == '''Vanillin''' == |
[[Image:Vanillin.png]] | [[Image:Vanillin.png]] | ||
| - | == Broader Implications == | + | == '''Broader Implications''' == |
| - | == Structural highlights and structure-function relationships == | + | == '''Structural highlights and structure-function relationships''' == |
| - | == Energy Transformation == | + | == '''Energy Transformation''' == |
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
| - | == References == | + | == '''References''' == |
<references/> | <references/> | ||
Revision as of 22:21, 29 November 2019
| This Sandbox is Reserved from Aug 26 through Dec 12, 2019 for use in the course CHEM 351 Biochemistry taught by Bonnie_Hall at the Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1556 through Sandbox Reserved 1575. |
To get started:
More help: Help:Editing |
Lignostilbene-α,ß-dioxygenase A structural features and important functional residues
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Kuatsjah E, Verstraete MM, Kobylarz MJ, Liu AKN, Murphy MEP, Eltis LD. Identification of functionally important residues and structural features in a bacterial lignostilbene dioxygenase. J Biol Chem. 2019 Jul 10. pii: RA119.009428. doi: 10.1074/jbc.RA119.009428. PMID:31292192 doi:http://dx.doi.org/10.1074/jbc.RA119.009428