Tetrahydroprotoberbine N-methyltransferase is a protein thats dimer interface includes six salt 6 salt bridges and 8 hydrogens bonds. It is expressed in E. coli and crystallized at a pH of 7.0. The crystals were grown in the presence of SAH,SAM, and SAH+SMS. Below are the two different substrates that were in the presence of the crystallized protein.
Function
The protein being studied, Tetrahydroprotoberbine N-methyltransferase, is found in yellow horned poppy (Glaucium Flavum). The function of the protein is substate recognition as well as catalysis for the ration engineering of enyzmes for chemoenzymatic synthesis and metabolic engineering. The relative activity of about 8 substrates were tested wiht Protoberberine having the highest percentage. GfTNMT's activity depends on temperature and pH. When the enzyme's activity was at a pH of 8, dropped 10% in activity. 10% activity was also dropped when the temperature was at 30 degrees Celsius. When at 4 degrees Celsius, the activity dropped even more down to 40%.
Mutants
In TMNT, three amino acid residues in the alpha14-helix form one side of the defining the BP region. The binding pocket consists of His-328(green), Ile-329(purple), and Phe-332(orange). The H328 mutation decreases in activity with stylopine and scoulerine producing a 5- and 2-fold while the activity with THP increases 2-fold.
Relevance
Studying Tetrahydroprotoberbine will provide commercial application where one will gain a lot of knowledge from both the research paper and online sources. Studying this protein will allow readers to engage in the material and apply their own knowledge to better understand the study. This research will provide descriptive roles that TNMT plays such as pathway leading to the formation of different substrates including Protoberberine. [1]
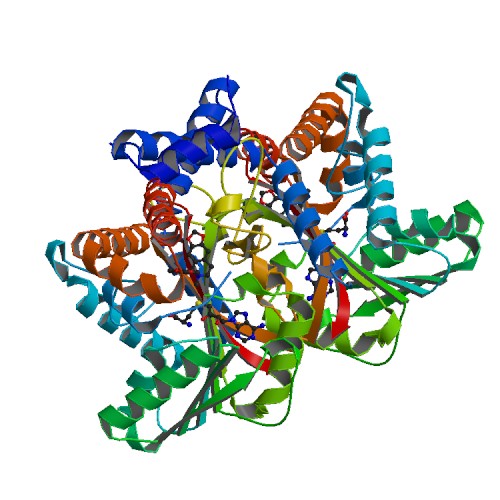
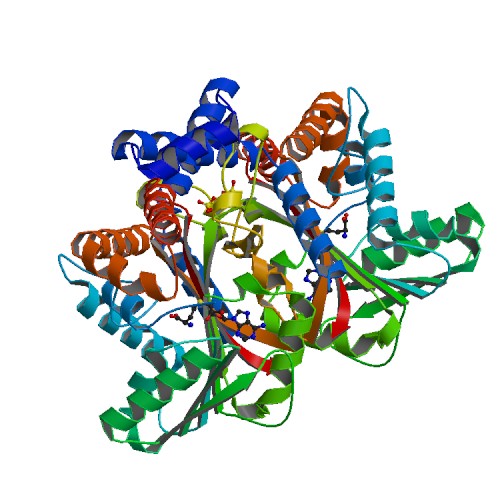
Structural Highlights
This scene displays the catalytic triad which are the amino acids His-208, Glu-204, and Glu-207. The authors explained within the paper that other amino acids may play a role in the triad as well. They were unsure but those three were the most accurate. These amino acids play an important role in catalysis for the protein.
This scene displays the spacefill view of Tetrahydroprotoberbine. This view allows viewers to see the different colored elements such as carbon(grey), nitrogen(blue), and oxygen(red).
This scene displays the ligand of the protein. The ligand of the protein is named SAM.
This scene displays the active site of my the protein. In green, there is Alanine, in yellow Asparatic Acid, and in blue Valine.


