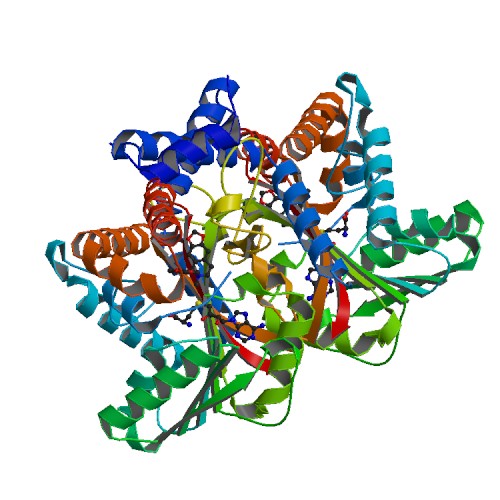
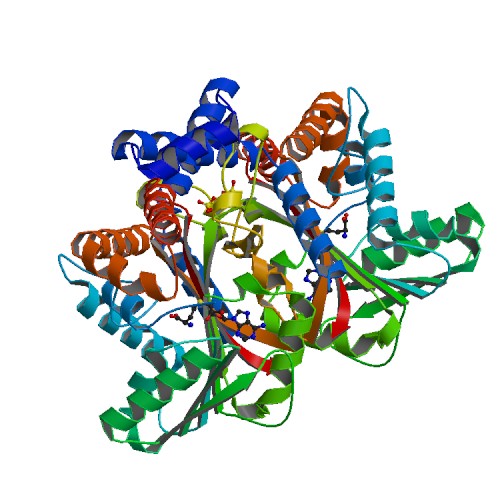
Tetrahydroprotoberberine N-methyltransferase
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
Studying Tetrahydroprotoberbine will provide commercial application where one will gain a lot of knowledge from both the research paper and online sources. Studying this protein will allow readers to engage in the material and apply their own knowledge to better understand the study. This research will provide descriptive roles that TNMT plays such as pathway leading to the formation of different substrates including Protoberberine. <ref>20069275 </ref> | Studying Tetrahydroprotoberbine will provide commercial application where one will gain a lot of knowledge from both the research paper and online sources. Studying this protein will allow readers to engage in the material and apply their own knowledge to better understand the study. This research will provide descriptive roles that TNMT plays such as pathway leading to the formation of different substrates including Protoberberine. <ref>20069275 </ref> | ||
= Structural Highlights = | = Structural Highlights = | ||
| - | This protein has a <scene name='82/829888/Catalytic_triad/7'>catalytic triad</scene> which consists of amino acids His-208, Glu-204, and Glu-207. The authors explained within the paper that other amino acids may play a role in the triad as well. They were unsure but those three were the most accurate. These amino acids play an important role in catalysis for the protein. <ref> 20069275 </ref> The basic <scene name='82/829888/Spacefill_view_of_protein/1'>spacefill view</scene> of the entire protein alllows readers to visualize the different elements show in different colors. The elements shown are carbons(grey), nitrogen(blue), and oxygen(red). This protein has a <scene name='82/829888/Ligand/1'> | + | This protein has a <scene name='82/829888/Catalytic_triad/7'>catalytic triad</scene> which consists of amino acids His-208, Glu-204, and Glu-207. The authors explained within the paper that other amino acids may play a role in the triad as well. They were unsure but those three were the most accurate. These amino acids play an important role in catalysis for the protein. <ref> 20069275 </ref> The basic <scene name='82/829888/Spacefill_view_of_protein/1'>spacefill view</scene> of the entire protein alllows readers to visualize the different elements show in different colors. The elements shown are carbons(grey), nitrogen(blue), and oxygen(red). This protein has a <scene name='82/829888/Ligand/1'>ligand</scene> which is SAM. There are <scene name='82/829888/Hydrophilic_side_chains/1'>hydrophilic side chains</scene>of SAM that form a small catalytic pocket and surrounds the amino group and methyl donor of SAM. This catalytic pocket forms a L shape. In green Glu-204, yellow is Glu-207, red is His-208, and Tyr-81 is blue. The <scene name='82/829888/Active_site/3'>active site</scene> of the protein consists of amino acids Valine-188(yellow), Aspartic Acid-187(blue), and Alanine-186(green), with purple being the rest of the ligand, SAM. The active site is the region where substrate molecules bind and undergo a chemical reaction. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
Revision as of 15:53, 30 November 2019
Structure
| |||||||||||