We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Protein phosphatase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
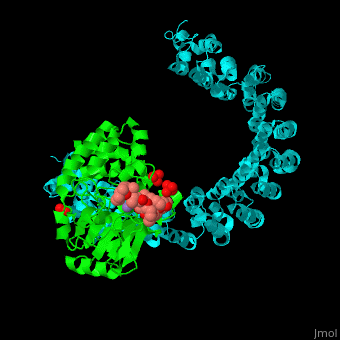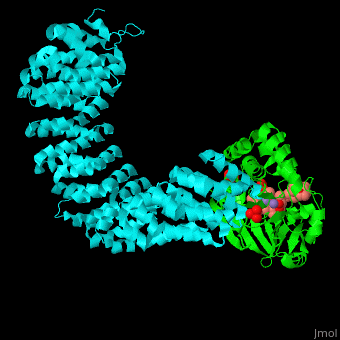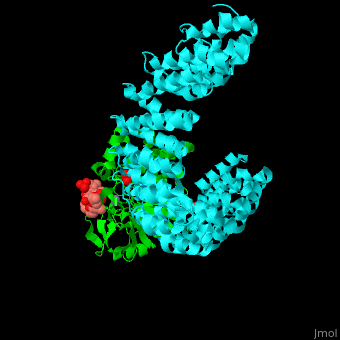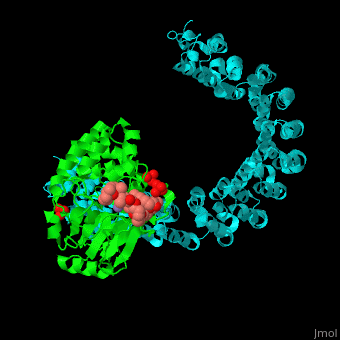
<StructureSection load='' size='350' side='right' scene='54/540142/Cv/1' caption='Human PP2A catalytic (green) and regulatory (cyan) subunits complex with tumor-inducing toxin and sulfate [[3k7v]]'> | <StructureSection load='' size='350' side='right' scene='54/540142/Cv/1' caption='Human PP2A catalytic (green) and regulatory (cyan) subunits complex with tumor-inducing toxin and sulfate [[3k7v]]'> | ||
| + | __TOC__ | ||
| + | |||
== Function == | == Function == | ||
'''Protein phosphatases''' (PP) or '''serine/threonine protein phosphatase''' regulate protein phosphorylation and thus are key in intracellular signal transduction processes.<br /> | '''Protein phosphatases''' (PP) or '''serine/threonine protein phosphatase''' regulate protein phosphorylation and thus are key in intracellular signal transduction processes.<br /> | ||
| Line 15: | Line 17: | ||
*<scene name='54/540142/Cv/6'>Mn+2 ion cofactors coordination site</scene>. | *<scene name='54/540142/Cv/6'>Mn+2 ion cofactors coordination site</scene>. | ||
*<scene name='54/540142/Cv/7'>Whole binding site</scene>. | *<scene name='54/540142/Cv/7'>Whole binding site</scene>. | ||
| + | == 3D Structures of protein phosphatase== | ||
| + | [[Protein phosphatase 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
== 3D Structures of protein phosphatase== | == 3D Structures of protein phosphatase== | ||
| Line 30: | Line 35: | ||
**[[3n5u]] – hPP1A catalytic subunit + retinoblastoma-associated protein<br /> | **[[3n5u]] – hPP1A catalytic subunit + retinoblastoma-associated protein<br /> | ||
**[[4g9j]], [[5ioh]] – hPP1A catalytic subunit + peptide<br /> | **[[4g9j]], [[5ioh]] – hPP1A catalytic subunit + peptide<br /> | ||
| + | **[[6b67]] – hPP1A catalytic subunit (mutant) + peptide<br /> | ||
*Protein phosphatase 1G | *Protein phosphatase 1G | ||
| Line 38: | Line 44: | ||
**[[4da1]] - hPP1K + Mn<br /> | **[[4da1]] - hPP1K + Mn<br /> | ||
| + | **[[6ak7]] – hPP1K (mutant) + Mg<br /> | ||
| - | *Protein phosphatase 2A | + | *Protein phosphatase 2A or serine/threonine-protein phosphatase 2A |
| - | **[[1b3u]], [[2g62]], [[2hv6]] – hPP2A regulatory subunit <br /> | + | **[[1b3u]], [[2g62]], [[2hv6]], [[4mew]], [[4i5k]], [[4i5j]], [[2jak]], [[2ixm]], [[2hv7] – hPP2A regulatory subunit <br /> |
| + | **[[5swf]], [[5k6s]], [[5jja]] – hPP2A regulatory subunit + BUBR1<br /> | ||
| + | **[[5sw9]] – hPP2A regulatory subunit + REPOMAN<br /> | ||
| + | **[[2pkg]], [[2pf4]] – hPP2A regulatory subunit + small T antigen<br /> | ||
**[[2ie3]], [[2ie4]], [[2npp]], [[2nyl]], [[2nym]] – hPP2A catalytic + regulatory subunit + tumor-inducing toxin<br /> | **[[2ie3]], [[2ie4]], [[2npp]], [[2nyl]], [[2nym]] – hPP2A catalytic + regulatory subunit + tumor-inducing toxin<br /> | ||
**[[3c5w]] – hPP2A catalytic + regulatory subunit + PP2A-specific methyltransferase<br /> | **[[3c5w]] – hPP2A catalytic + regulatory subunit + PP2A-specific methyltransferase<br /> | ||
| + | **[[3p71]], [[2iae]] – hPP2A catalytic + regulatory subunit + LCMT-1 <br /> | ||
| + | **[[3dw8]] – hPP2A catalytic + regulatory subunit (mutant) + LCMT-1 <br /> | ||
| + | **[[3fga]] – hPP2A catalytic + regulatory subunit + LCMT-1 + SGO<br /> | ||
| + | **[[4i5n]], [[4i5l]] – hPP2A catalytic + regulatory subunit + MCLR<br /> | ||
| + | **[[3k7w]] – hPP2A catalytic + regulatory subunit + toxin<br /> | ||
| + | **[[4iyp]] – hPP2A catalytic subunit + immunoglobin-binding protein<br /> | ||
| + | **[[6ef4]] – mPP2A (mutant) - mouse<br /> | ||
| + | **[[5w0w]] – mPP2A + TIPRL<br /> | ||
*Protein phosphatase 2B See [[Calcineurin]] | *Protein phosphatase 2B See [[Calcineurin]] | ||
| Line 52: | Line 70: | ||
**[[4raf]], [[4rag]] - hPP2C α (mutant) + Mn<br /> | **[[4raf]], [[4rag]] - hPP2C α (mutant) + Mn<br /> | ||
**[[3d8k]] – PP2C – ''Toxoplasma gondii''<br /> | **[[3d8k]] – PP2C – ''Toxoplasma gondii''<br /> | ||
| - | **[[3jrq]], [[3nmn]] – AtPP2C + Pyl1 + pyrabactin | + | **[[3ujk]] – AtPP2C - ''Arabidopsis thaliana'' <br /> |
| + | **[[4yzg]] – AtPP2C (mutant) <br /> | ||
| + | **[[3jrq]], [[3nmn]] – AtPP2C + Pyl1 + pyrabactin <br /> | ||
**[[3kdj]] - AtPP2C + Pyl1 + abscicic acid<br /> | **[[3kdj]] - AtPP2C + Pyl1 + abscicic acid<br /> | ||
**[[3nmt]], [[3kb3]], [[3nmv]], [[3ujl]] – AtPP2C + Pyl2<br /> | **[[3nmt]], [[3kb3]], [[3nmv]], [[3ujl]] – AtPP2C + Pyl2<br /> | ||
**[[4la7]], [[4lg5]], [[4lga]], [[4lgb]] – AtPP2C + Pyl2 + ligand<br /> | **[[4la7]], [[4lg5]], [[4lga]], [[4lgb]] – AtPP2C + Pyl2 + ligand<br /> | ||
| + | **[[5vr7]], [[5vro]], [[5vs5]], [[5vsq]], [[5vsr]], [[5vt7]] – AtPP2C + Pyl2 + quinolone derivative<br /> | ||
**[[4ds8]], [[5jo1]], [[5jo2]] – AtPP2C + Pyl3 + Mn<br /> | **[[4ds8]], [[5jo1]], [[5jo2]] – AtPP2C + Pyl3 + Mn<br /> | ||
**[[3rt0]] – AtPP2C (mutant) + Pyl10<br /> | **[[3rt0]] – AtPP2C (mutant) + Pyl10<br /> | ||
**[[4n0g]] – AtPP2C + Pyl13<br /> | **[[4n0g]] – AtPP2C + Pyl13<br /> | ||
**[[3qn1]], [[3zvu]], [[4wvo]] – AtPP2C + Pyr1<br /> | **[[3qn1]], [[3zvu]], [[4wvo]] – AtPP2C + Pyr1<br /> | ||
| - | **[[ | + | **[[5or2]], [[5or6]] – AtPP2C + Pyr1 + abscicic acid analog<br /> |
| - | + | ||
**[[4yzh]] – AtPP2C (mutant) + chlorophyll-binding protein peptide<br /> | **[[4yzh]] – AtPP2C (mutant) + chlorophyll-binding protein peptide<br /> | ||
**[[3ujg]] - AtPP2C + SRK2E<br /> | **[[3ujg]] - AtPP2C + SRK2E<br /> | ||
| + | **[[5mn0]] – AtPP2C + ABA receptor <br /> | ||
*Protein phosphatase 4 | *Protein phosphatase 4 | ||
Revision as of 08:36, 2 December 2019
| |||||||||||
3D Structures of protein phosphatase
Updated on 02-December-2019 {{#tree:id=OrganizedByTopic|openlevels=0|
- Protein phosphatase 1
- 2rlt - PP1 regulatory subunit - pig - NMR
- 2rlt - PP1 regulatory subunit - pig - NMR
- Protein phosphatase 1A
- Protein phosphatase 1G
- Protein phosphatase 1K
- Protein phosphatase 2A or serine/threonine-protein phosphatase 2A
- 1b3u, 2g62, 2hv6, 4mew, 4i5k, 4i5j, 2jak, 2ixm, [[2hv7] – hPP2A regulatory subunit
- 5swf, 5k6s, 5jja – hPP2A regulatory subunit + BUBR1
- 5sw9 – hPP2A regulatory subunit + REPOMAN
- 2pkg, 2pf4 – hPP2A regulatory subunit + small T antigen
- 2ie3, 2ie4, 2npp, 2nyl, 2nym – hPP2A catalytic + regulatory subunit + tumor-inducing toxin
- 3c5w – hPP2A catalytic + regulatory subunit + PP2A-specific methyltransferase
- 3p71, 2iae – hPP2A catalytic + regulatory subunit + LCMT-1
- 3dw8 – hPP2A catalytic + regulatory subunit (mutant) + LCMT-1
- 3fga – hPP2A catalytic + regulatory subunit + LCMT-1 + SGO
- 4i5n, 4i5l – hPP2A catalytic + regulatory subunit + MCLR
- 3k7w – hPP2A catalytic + regulatory subunit + toxin
- 4iyp – hPP2A catalytic subunit + immunoglobin-binding protein
- 6ef4 – mPP2A (mutant) - mouse
- 5w0w – mPP2A + TIPRL
- 1b3u, 2g62, 2hv6, 4mew, 4i5k, 4i5j, 2jak, 2ixm, [[2hv7] – hPP2A regulatory subunit
- Protein phosphatase 2B See Calcineurin
- Protein phosphatase 2C
- 2iq1 – hPP2C κ
- 4raf, 4rag - hPP2C α (mutant) + Mn
- 3d8k – PP2C – Toxoplasma gondii
- 3ujk – AtPP2C - Arabidopsis thaliana
- 4yzg – AtPP2C (mutant)
- 3jrq, 3nmn – AtPP2C + Pyl1 + pyrabactin
- 3kdj - AtPP2C + Pyl1 + abscicic acid
- 3nmt, 3kb3, 3nmv, 3ujl – AtPP2C + Pyl2
- 4la7, 4lg5, 4lga, 4lgb – AtPP2C + Pyl2 + ligand
- 5vr7, 5vro, 5vs5, 5vsq, 5vsr, 5vt7 – AtPP2C + Pyl2 + quinolone derivative
- 4ds8, 5jo1, 5jo2 – AtPP2C + Pyl3 + Mn
- 3rt0 – AtPP2C (mutant) + Pyl10
- 4n0g – AtPP2C + Pyl13
- 3qn1, 3zvu, 4wvo – AtPP2C + Pyr1
- 5or2, 5or6 – AtPP2C + Pyr1 + abscicic acid analog
- 4yzh – AtPP2C (mutant) + chlorophyll-binding protein peptide
- 3ujg - AtPP2C + SRK2E
- 5mn0 – AtPP2C + ABA receptor
- 2iq1 – hPP2C κ
- Protein phosphatase 4
- 4wsf – PP4 regulatory subunit + Cenp-C – Drosophila melanogaster
- 4wsf – PP4 regulatory subunit + Cenp-C – Drosophila melanogaster
- Protein phosphatase 5
- 1wao – hPP5 + Mn
- 5muf – hPP5
- 1a17 – hPP5 protein-interacting domain
- 2bug – hPP5 protein-interacting domain (mutant) + Hsp90 peptide - NMR
- 1s95, 3h60 – hPP5 catalytic domain + Mn
- 3h61, 3h62, 3h63, 3h64, 3h66, 3h67, 3h68, 3h69, 4zvz, 4zx2, 4zvz, 4zx2 – hPP5 catalytic domain + inhibitor + Mn
- 5hpe – hPP5 catalytic domain/Hsp90 peptide + Mn
- 4ja7, 4ja9 - rPP5 catalytic domain + inhibitor
- 3icf - yPP5 catalytic domain + Fe - yeast
- 5jjt – AtPP5 + Ni
- 1wao – hPP5 + Mn
- Protein phosphatase
}}
References
- ↑ Ragolia L, Begum N. Protein phosphatase-1 and insulin action. Mol Cell Biochem. 1998 May;182(1-2):49-58. PMID:9609113
- ↑ Resjo S, Goransson O, Harndahl L, Zolnierowicz S, Manganiello V, Degerman E. Protein phosphatase 2A is the main phosphatase involved in the regulation of protein kinase B in rat adipocytes. Cell Signal. 2002 Mar;14(3):231-8. PMID:11812651
- ↑ Lipinszki Z, Lefevre S, Savoian MS, Singleton MR, Glover DM, Przewloka MR. Centromeric binding and activity of Protein Phosphatase 4. Nat Commun. 2015 Jan 6;6:5894. doi: 10.1038/ncomms6894. PMID:25562660 doi:http://dx.doi.org/10.1038/ncomms6894
- ↑ Chinkers M. Protein phosphatase 5 in signal transduction. Trends Endocrinol Metab. 2001 Jan-Feb;12(1):28-32. PMID:11137038
- ↑ Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 2013 May;14(6):e229-38. doi: 10.1016/S1470-2045(12)70558-2. PMID:23639323 doi:http://dx.doi.org/10.1016/S1470-2045(12)70558-2
- ↑ Rudrabhatla P, Pant HC. Role of protein phosphatase 2A in Alzheimer's disease. Curr Alzheimer Res. 2011 Sep;8(6):623-32. PMID:21605044
- ↑ Huhn J, Jeffrey PD, Larsen K, Rundberget T, Rise F, Cox NR, Arcus V, Shi Y, Miles CO. A structural basis for the reduced toxicity of dinophysistoxin-2. Chem Res Toxicol. 2009 Nov;22(11):1782-6. PMID:19916524 doi:10.1021/tx9001622
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Alexsandra Tifane Santos do Nascimento, Joel L. Sussman