We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1558
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Function(s) and Biological Relevance == | == Function(s) and Biological Relevance == | ||
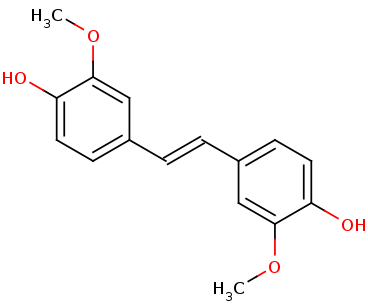
| - | Lignostilbene-,-dioxygenase | + | Lignostilbene-α,β-dioxygenase (LsdA) from the bacterium |
''''Sphingomonas paucimobilis''''. It is a nonheme iron oxygenase that catalyzes the cleavage of lignostilbene, a compound | ''''Sphingomonas paucimobilis''''. It is a nonheme iron oxygenase that catalyzes the cleavage of lignostilbene, a compound | ||
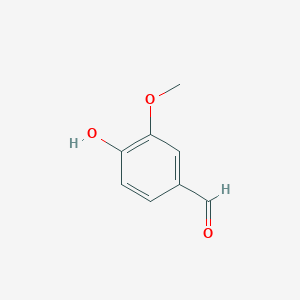
arising in lignin transformation, to two vanillin molecules. The substrate for this enzyme is lignostilbene. Phenylazophenol | arising in lignin transformation, to two vanillin molecules. The substrate for this enzyme is lignostilbene. Phenylazophenol | ||
| Line 18: | Line 18: | ||
tested on several substituted stilbenes, LsdA exhibited the greatest specificity for lignostilbene. These experiments further indicated that the substrate’s | tested on several substituted stilbenes, LsdA exhibited the greatest specificity for lignostilbene. These experiments further indicated that the substrate’s | ||
4-hydroxy moiety is required for catalysis and that this moiety | 4-hydroxy moiety is required for catalysis and that this moiety | ||
| - | cannot be replaced with a methoxy group. This | + | cannot be replaced with a methoxy group. This expands our |
mechanistic understanding of LsdA and related stilbene-cleaving dioxygenases. | mechanistic understanding of LsdA and related stilbene-cleaving dioxygenases. | ||
Revision as of 00:59, 9 December 2019
| This Sandbox is Reserved from Aug 26 through Dec 12, 2019 for use in the course CHEM 351 Biochemistry taught by Bonnie_Hall at the Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1556 through Sandbox Reserved 1575. |
To get started:
More help: Help:Editing |
Lignostilbene-α,β-dioxygenase A (LsdA) Catalyzation
| |||||||||||
References
Kuatsjah, Eugene, et al. “Identification of Functionally Important Residues and Structural Features in a Bacterial Lignostilbene Dioxygenase.” Journal of Biological Chemistry, vol. 294, no. 35, 2019, pp. 12911–12920., doi:10.1074/jbc.ra119.009428.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644