We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1558
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
mechanistic understanding of LsdA and related stilbene-cleaving dioxygenases. | mechanistic understanding of LsdA and related stilbene-cleaving dioxygenases. | ||
== Structural highlights and structure-function relationships == | == Structural highlights and structure-function relationships == | ||
| - | The <scene name='82/823082/Catalytic_triad/2'>Catalytic Triad</scene> of this protein is primarily made of the amino acids that are the main factor in catalysis. The 3 amino acids are Phe-59, Tyr101, and Lys-134. The <scene name='82/823082/Colored_secondary/1'>secondary and terteriary structure</scene> is a fold of LsdA of a seven-bladed -propeller, typical of the carotenoid cleavage oxygenates (CCO's), which usually catalyze the oxidative cleavage of a double bond in carotenoids. | + | The <scene name='82/823082/Catalytic_triad/2'>Catalytic Triad</scene> of this protein is primarily made of the amino acids that are the main factor in catalysis. The 3 amino acids are Phe-59, Tyr101, and Lys-134. The <scene name='82/823082/Colored_secondary/1'>secondary and terteriary structure</scene> is a fold of LsdA of a seven-bladed -propeller, typical of the carotenoid cleavage oxygenates (CCO's), which usually catalyze the oxidative cleavage of a double bond in carotenoids<ref>PMID 31292192</ref>. |
The structures also consist of α-helices and ß-sheets. The <scene name='82/823082/Hydrogen_bonding/1'>active site</scene> occurs at the center of the propeller and contains an Fe2+. The <scene name='82/823082/Hydrophobicity_whole_protein/1'>hydrophobicity</scene> and <scene name='82/823082/Spacefill_whole_protein/1'>spacefill</scene> view of the ligand in the protein, which shows that both hydrophilic and hydrophobic residues are important to the ligand in the binding site. | The structures also consist of α-helices and ß-sheets. The <scene name='82/823082/Hydrogen_bonding/1'>active site</scene> occurs at the center of the propeller and contains an Fe2+. The <scene name='82/823082/Hydrophobicity_whole_protein/1'>hydrophobicity</scene> and <scene name='82/823082/Spacefill_whole_protein/1'>spacefill</scene> view of the ligand in the protein, which shows that both hydrophilic and hydrophobic residues are important to the ligand in the binding site. | ||
Revision as of 02:58, 9 December 2019
| This Sandbox is Reserved from Aug 26 through Dec 12, 2019 for use in the course CHEM 351 Biochemistry taught by Bonnie_Hall at the Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1556 through Sandbox Reserved 1575. |
To get started:
More help: Help:Editing |
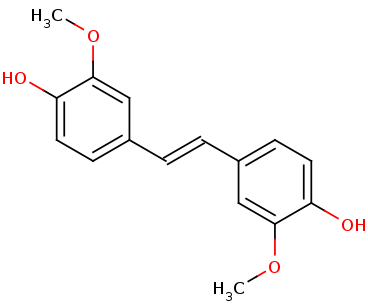
Lignostilbene-α,β-dioxygenase A (LsdA) Catalyzation
| |||||||||||