We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Krebs cycle step 1
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
<p>Figure: Mechanism of the citrate formation</p> | <p>Figure: Mechanism of the citrate formation</p> | ||
| - | <p>In the first reaction (see Figure) of the Krebs cycle, acetyl-CoA reacts with oxal acetate. | + | <p>In the first reaction (see Figure) of the [[Citric Acid Cycle|Krebs cycle]], acetyl-CoA reacts with oxal acetate. |
Acetyl-CoA is coupled (yellow background) with the C2 atom of the oxal acetate and the C6 body | Acetyl-CoA is coupled (yellow background) with the C2 atom of the oxal acetate and the C6 body | ||
citryl-CoA is formed. This is a high-energy thioester bound in the enzyme, and it is not released. | citryl-CoA is formed. This is a high-energy thioester bound in the enzyme, and it is not released. | ||
In the second step of this reaction, the energy-rich thioester is hydrolyzed, and citrate and the coenzyme A (CoA-SH) were formed.</p> | In the second step of this reaction, the energy-rich thioester is hydrolyzed, and citrate and the coenzyme A (CoA-SH) were formed.</p> | ||
<p>The mechanism of the citrate synthase prevents premature and undesirable hydrolysis of acetyl-CoA, and, consequently, a waste of energy. </p> | <p>The mechanism of the citrate synthase prevents premature and undesirable hydrolysis of acetyl-CoA, and, consequently, a waste of energy. </p> | ||
Current revision
First step: Citrate Synthase
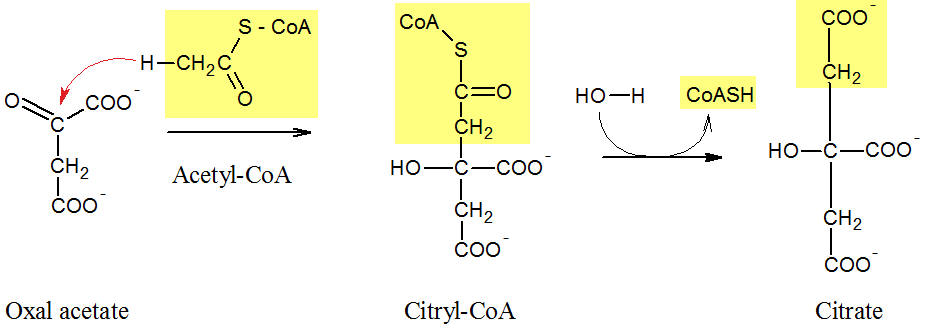
Figure: Mechanism of the citrate formation
In the first reaction (see Figure) of the Krebs cycle, acetyl-CoA reacts with oxal acetate. Acetyl-CoA is coupled (yellow background) with the C2 atom of the oxal acetate and the C6 body citryl-CoA is formed. This is a high-energy thioester bound in the enzyme, and it is not released. In the second step of this reaction, the energy-rich thioester is hydrolyzed, and citrate and the coenzyme A (CoA-SH) were formed.
The mechanism of the citrate synthase prevents premature and undesirable hydrolysis of acetyl-CoA, and, consequently, a waste of energy.