We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Krebs cycle step 6
From Proteopedia
(Difference between revisions)
m (Sandbox Oldenburg09 moved to Krebs cycle step 6) |
|||
| Line 1: | Line 1: | ||
| - | <h2>Step 6 of the Krebs cycle: Succinate Dehydrogenase</h2> | + | <h2>Step 6 of the Krebs cycle: [[Succinate Dehydrogenase]]</h2> |
[[Image:succinat_2.jpg]] | [[Image:succinat_2.jpg]] | ||
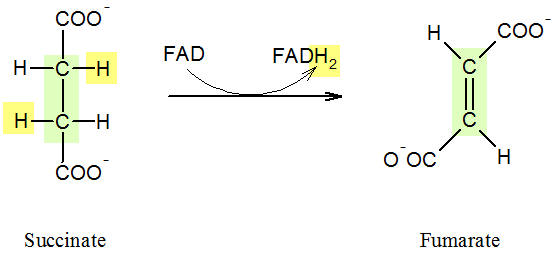
<p>Figure: Formation of Fumarate</p> | <p>Figure: Formation of Fumarate</p> | ||
| - | <p>In the sixth step, succinate is converted to fumarate; here, a dehydrogenation takes place because two protons are removed. | + | <p>In the sixth step, succinate is converted to fumarate; here, a dehydrogenation takes place because two protons are removed. FAD serves as coenzyme wich is bound covalently to the enzyme succinate dehydrogenase, so that usually the notation E-FAD is used. The liberated protons (in yellow) are taken up by FAD which is oxidised to FADH<sub>2</sub>. Unlike NADH, FADH<sub>2</sub> does not need to be |
funneled into the respiratory chain, but is reduced directly in the enzyme back to FAD, and the reaciton can take place with another succinate molecule. </p> | funneled into the respiratory chain, but is reduced directly in the enzyme back to FAD, and the reaciton can take place with another succinate molecule. </p> | ||
Current revision
Step 6 of the Krebs cycle: Succinate Dehydrogenase
Figure: Formation of Fumarate
In the sixth step, succinate is converted to fumarate; here, a dehydrogenation takes place because two protons are removed. FAD serves as coenzyme wich is bound covalently to the enzyme succinate dehydrogenase, so that usually the notation E-FAD is used. The liberated protons (in yellow) are taken up by FAD which is oxidised to FADH2. Unlike NADH, FADH2 does not need to be funneled into the respiratory chain, but is reduced directly in the enzyme back to FAD, and the reaciton can take place with another succinate molecule.