Molecular Playground/Taxol
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| + | <StructureSection load='' size='350' side='right' caption='Paclitaxel (also known as Taxol)' scene='Rohan_Patil/Sandbox1/Taxol/6'> | ||
<applet size=350 frame='true' align='right' | <applet size=350 frame='true' align='right' | ||
caption='Paclitaxel (also known as Taxol)' /> <scene name='Rohan_Patil/Sandbox1/Taxol/6'>Paclitaxel</scene> | caption='Paclitaxel (also known as Taxol)' /> <scene name='Rohan_Patil/Sandbox1/Taxol/6'>Paclitaxel</scene> | ||
| + | |||
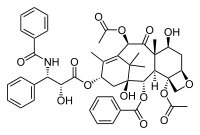
[[Image:Paclitaxel.png|frame|Paclitaxel (Taxol, Bristol-Myers Squibb)]] | [[Image:Paclitaxel.png|frame|Paclitaxel (Taxol, Bristol-Myers Squibb)]] | ||
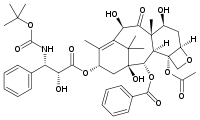
| - | [[Image:Docetaxel.png|frame|Docetaxel (Taxotere, Sanofi-aventis)]]Paclitaxel is one of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst and on display at the [http://www.molecularplayground.org/ Molecular Playground]. | + | [[Image:Docetaxel.png|frame|Docetaxel (Taxotere, Sanofi-aventis)]] |
| + | |||
| + | Paclitaxel is one of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst and on display at the [http://www.molecularplayground.org/ Molecular Playground]. | ||
Paclitaxel, also called Taxol (Bristol-Myers Squibb), is a plant derived anti-cancer agent that was first isolated from the bark of Pacific yew tree, ''Taxus brevifolia'', in 1971. It is a complex diterpenoid with a bulky, fused ring system as well as a number of hydrophobic substituents. Approved by the FDA in 1992, it is currently being used in the treatment of ovarian, breast and lung cancers. In addition, therapies are being developed for treatment of Alzheimer's and post-heart surgery patients. | Paclitaxel, also called Taxol (Bristol-Myers Squibb), is a plant derived anti-cancer agent that was first isolated from the bark of Pacific yew tree, ''Taxus brevifolia'', in 1971. It is a complex diterpenoid with a bulky, fused ring system as well as a number of hydrophobic substituents. Approved by the FDA in 1992, it is currently being used in the treatment of ovarian, breast and lung cancers. In addition, therapies are being developed for treatment of Alzheimer's and post-heart surgery patients. | ||
| Line 25: | Line 29: | ||
Molecular Playground Banner: "Docetaxel (Taxotere), an analaog of the plant derived anti-cancer agent paclitaxel" | Molecular Playground Banner: "Docetaxel (Taxotere), an analaog of the plant derived anti-cancer agent paclitaxel" | ||
| - | + | </StructureSection> | |
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 11:35, 30 December 2019
| |||||||||||
References
- ↑ Wilson SA, Cummings EM, Roberts SC. Multi-scale engineering of plant cell cultures for promotion of specialized metabolism. Curr Opin Biotechnol. 2014 Oct;29:163-70. doi: 10.1016/j.copbio.2014.07.001. Epub, 2014 Jul 24. PMID:25063984 doi:http://dx.doi.org/10.1016/j.copbio.2014.07.001
- ↑ Wilson SA, Roberts SC. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J. 2012 Apr;10(3):249-68. doi: 10.1111/j.1467-7652.2011.00664.x., Epub 2011 Nov 8. PMID:22059985 doi:http://dx.doi.org/10.1111/j.1467-7652.2011.00664.x
- ↑ Carvacho HB, Perez C, Zuniga G, Mahn A. Effect of methyl jasmonate, sodium selenate and chitosan as exogenous elicitors on the phenolic compounds profile of broccoli sprouts. J Sci Food Agric. 2014 Sep;94(12):2555-61. doi: 10.1002/jsfa.6596. Epub 2014 Mar , 18. PMID:24497113 doi:http://dx.doi.org/10.1002/jsfa.6596
- ↑ Gu XC, Chen JF, Xiao Y, Di P, Xuan HJ, Zhou X, Zhang L, Chen WS. Overexpression of allene oxide cyclase promoted tanshinone/phenolic acid production in Salvia miltiorrhiza. Plant Cell Rep. 2012 Dec;31(12):2247-59. doi: 10.1007/s00299-012-1334-9. Epub, 2012 Aug 29. PMID:22926031 doi:http://dx.doi.org/10.1007/s00299-012-1334-9
- ↑ Sabater-Jara AB, Onrubia M, Moyano E, Bonfill M, Palazon J, Pedreno MA, Cusido RM. Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus x media cell cultures. Plant Biotechnol J. 2014 Oct;12(8):1075-84. doi: 10.1111/pbi.12214. Epub 2014 Jun, 9. PMID:24909837 doi:http://dx.doi.org/10.1111/pbi.12214
- ↑ Sasaki Y, Asamizu E, Shibata D, Nakamura Y, Kaneko T, Awai K, Amagai M, Kuwata C, Tsugane T, Masuda T, Shimada H, Takamiya K, Ohta H, Tabata S. Monitoring of methyl jasmonate-responsive genes in Arabidopsis by cDNA macroarray: self-activation of jasmonic acid biosynthesis and crosstalk with other phytohormone signaling pathways. DNA Res. 2001 Aug 31;8(4):153-61. PMID:11572481
Proteopedia Page Contributors and Editors (what is this?)
Sarah Wilson, Alexander Berchansky, Rohan Patil, Elizabeth Cummings, Michal Harel, Lynmarie K Thompson